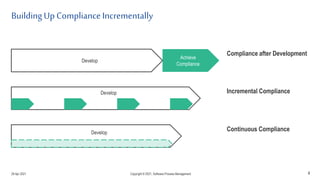
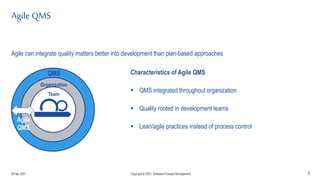
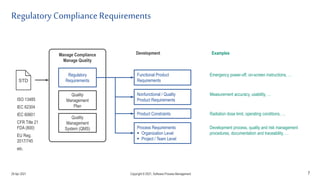
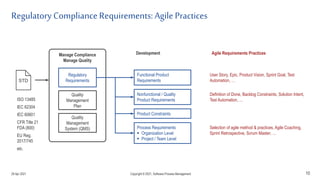
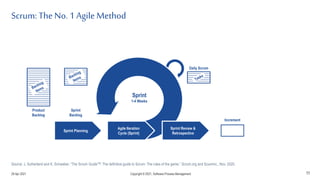
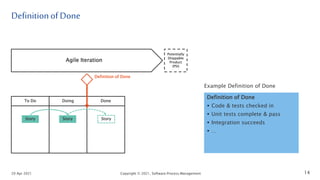
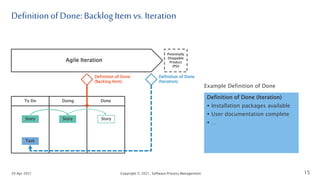
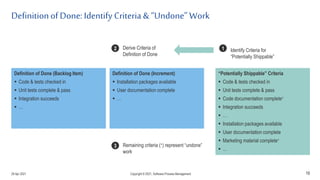
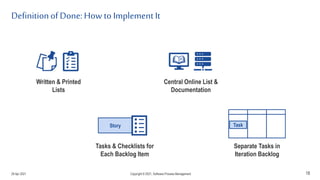
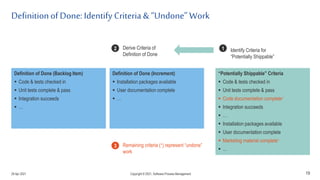
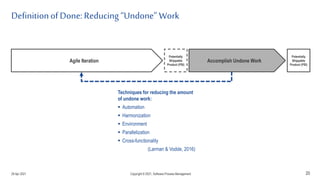
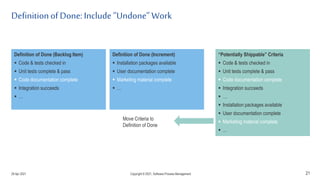
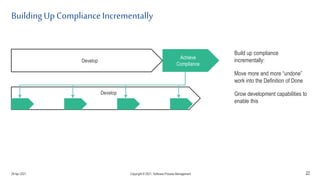
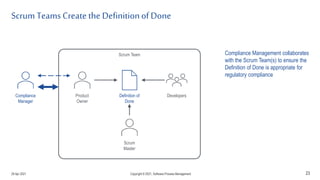
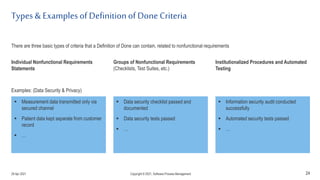
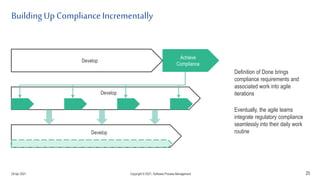
The document discusses applying agile practices to medical device development while maintaining regulatory compliance. It recommends building compliance incrementally by including more criteria in the Definition of Done for each iteration. This reduces "undone" work over time as agile teams integrate compliance work into their daily routines. Establishing an Agile Quality Management System and collaborating between compliance managers and agile teams can help infuse compliance practices into development.