Embed presentation
Download as PDF, PPTX





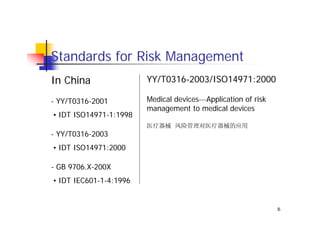











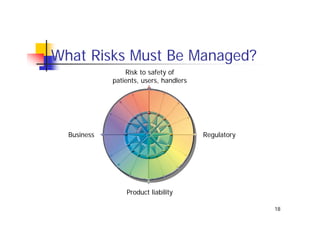
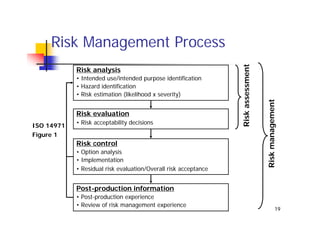


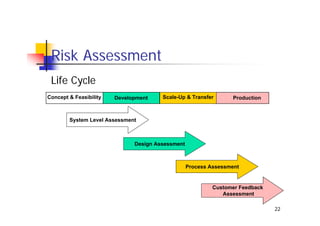
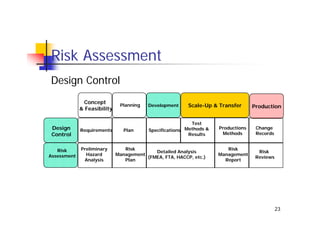

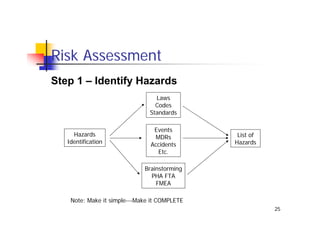

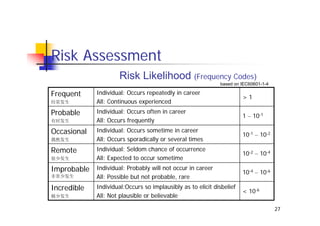
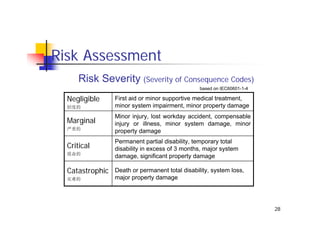
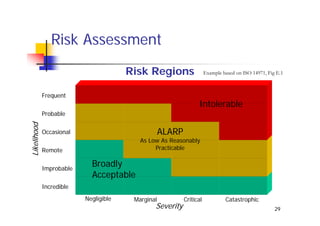
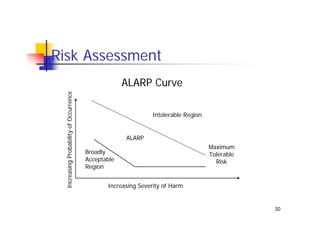



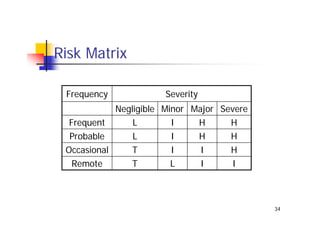
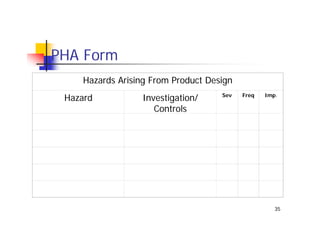


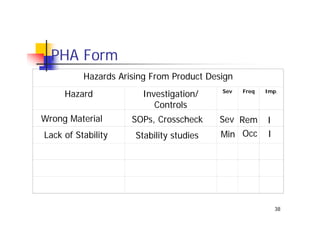
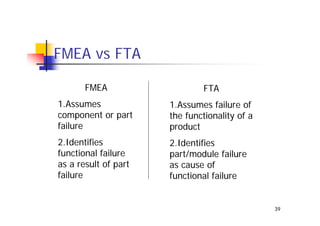
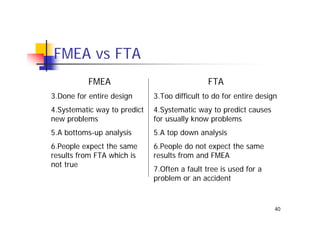


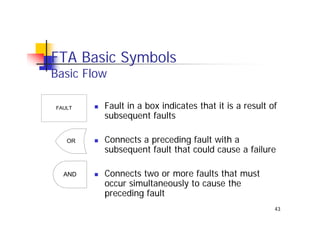
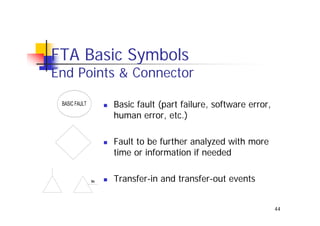
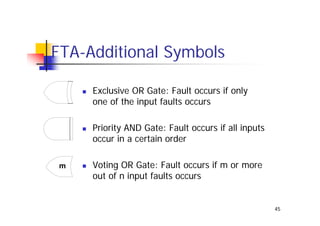
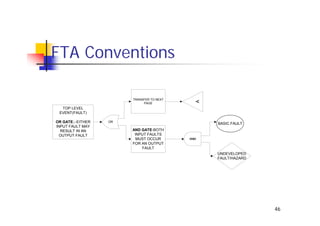
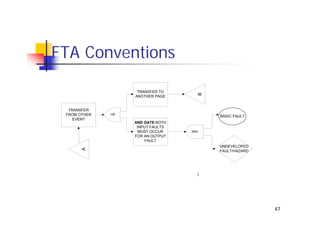



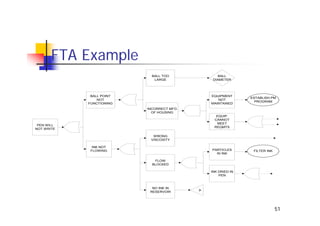
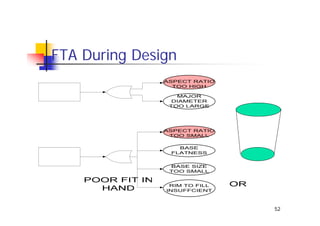
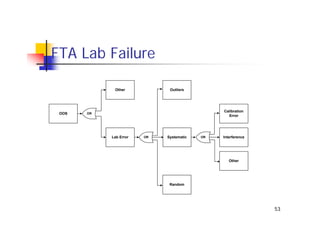
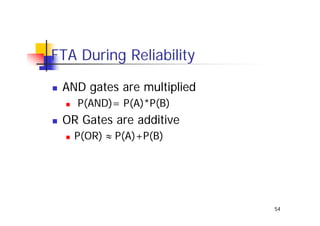
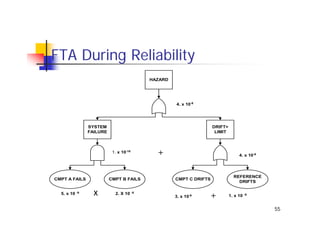



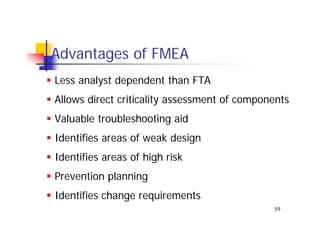
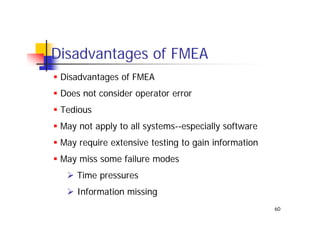







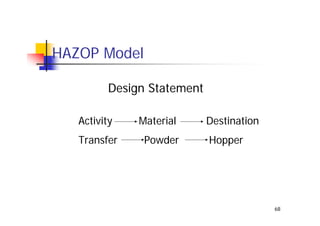
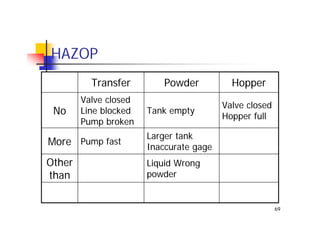
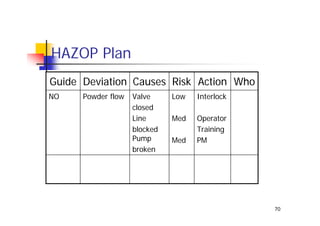


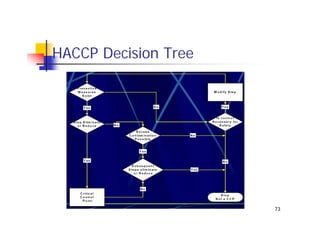
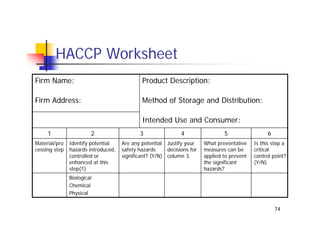
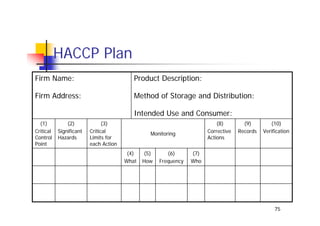





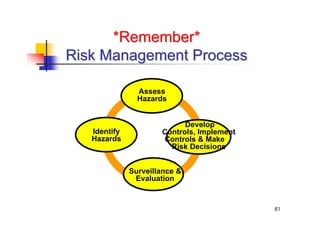









This document discusses risk management for medical devices. It defines key risk management terms and concepts. The risk management process involves risk assessment, analysis, evaluation, and control. Risk assessment tools include risk matrices, preliminary hazard analysis, fault tree analysis, failure mode and effects analysis, and hazard and operability analysis. Medical device risk management aims to ensure patient, user, and handler safety, as well as manage business and regulatory risks.

























































































