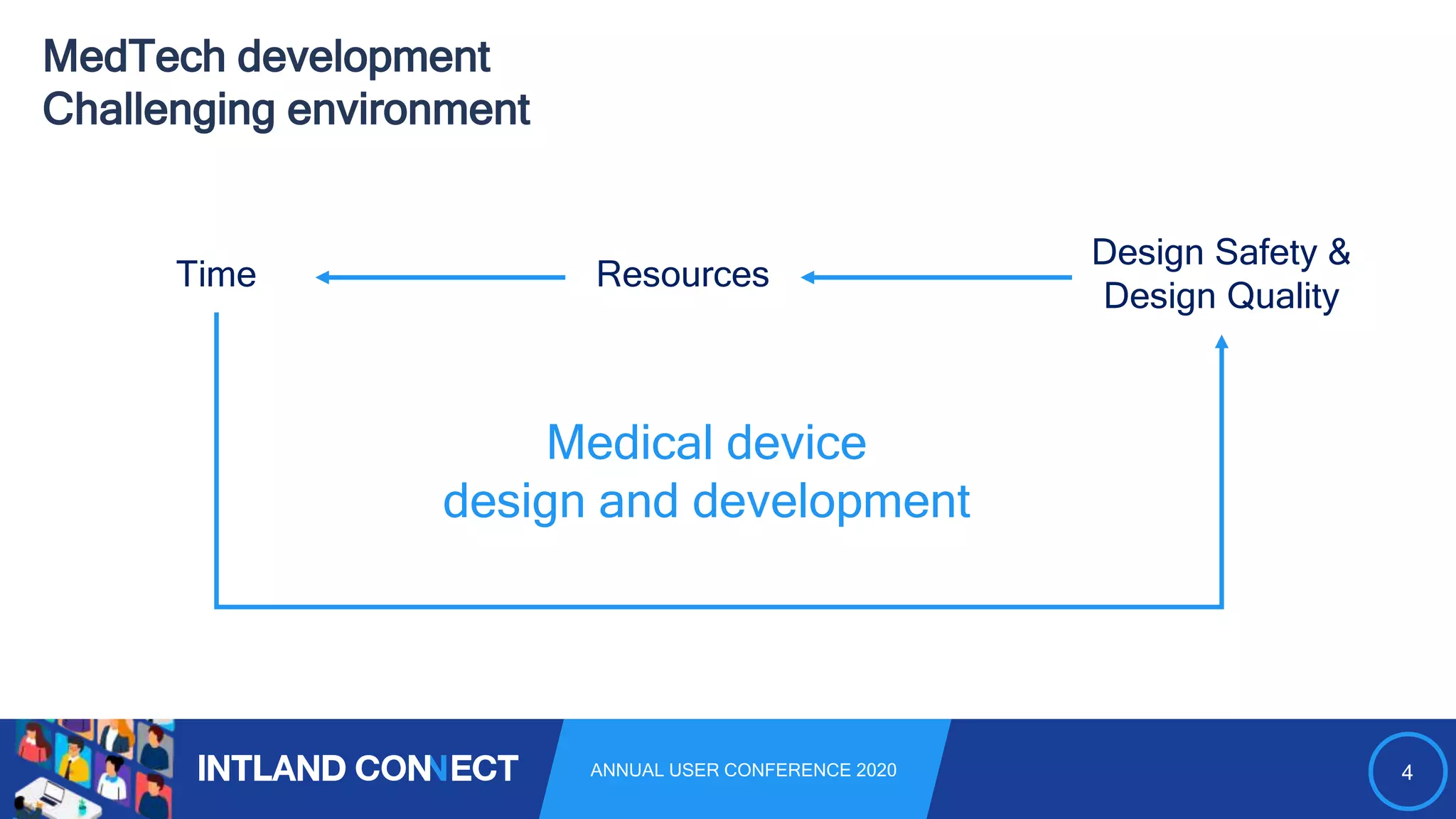
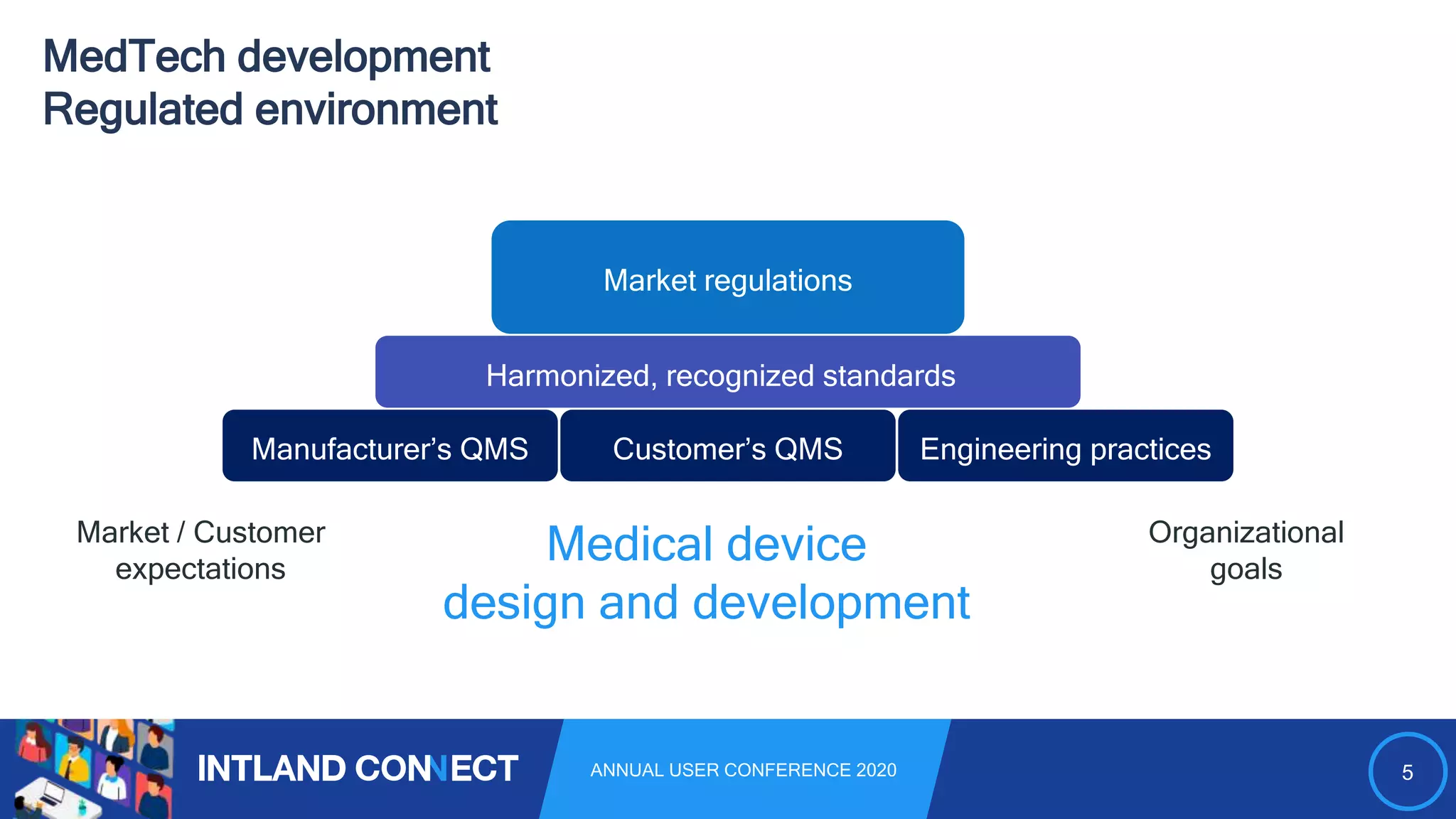
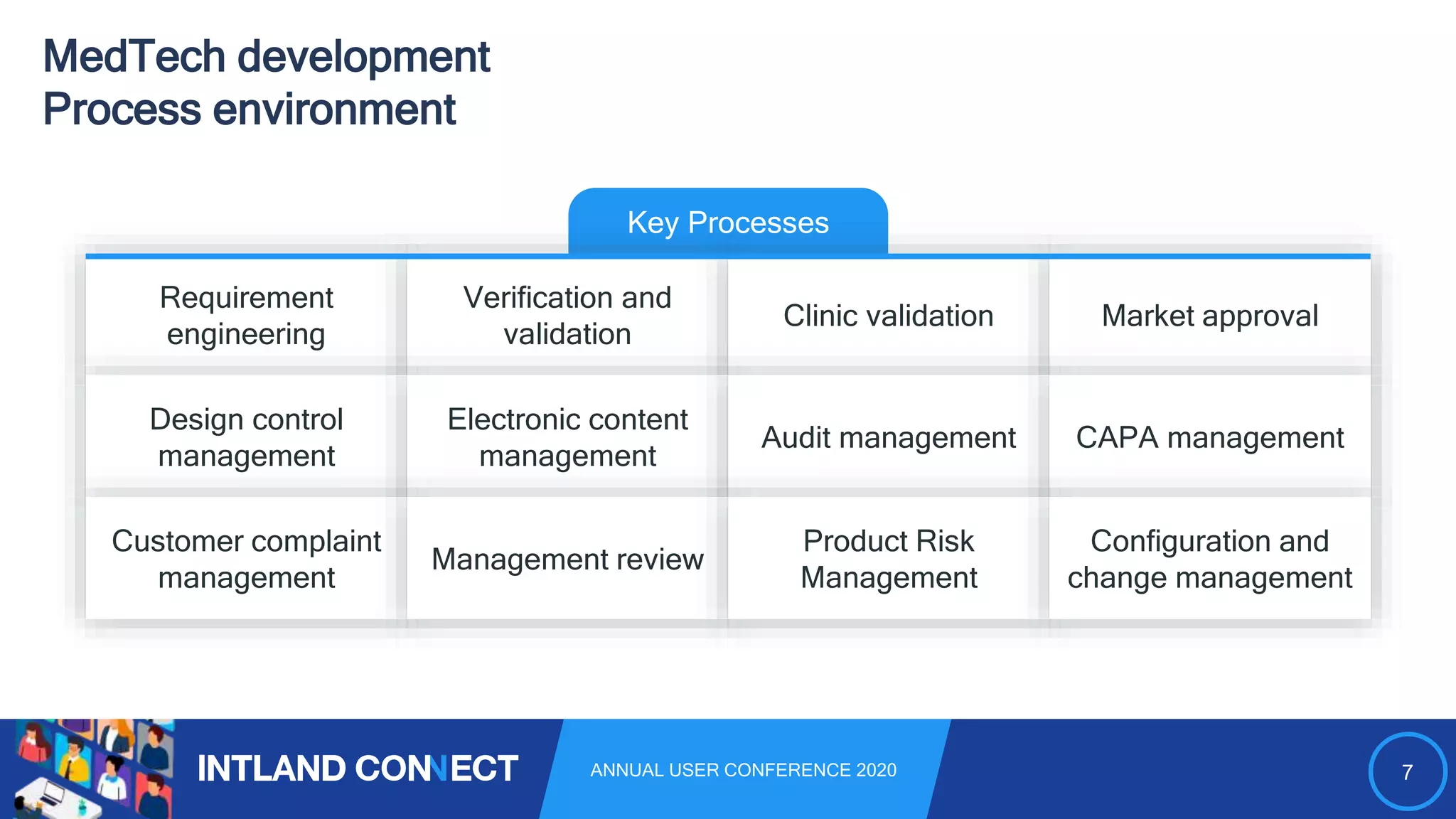
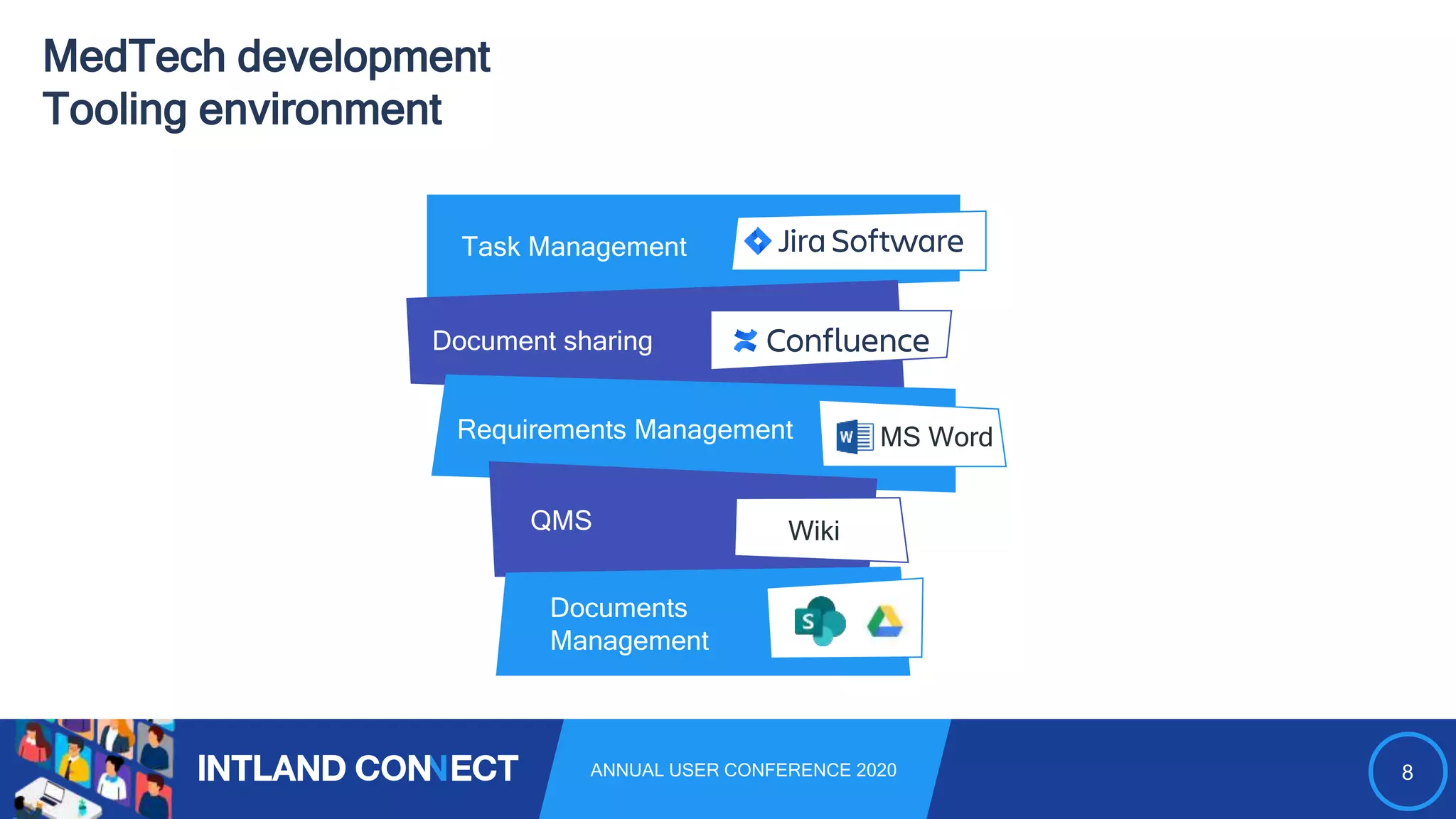
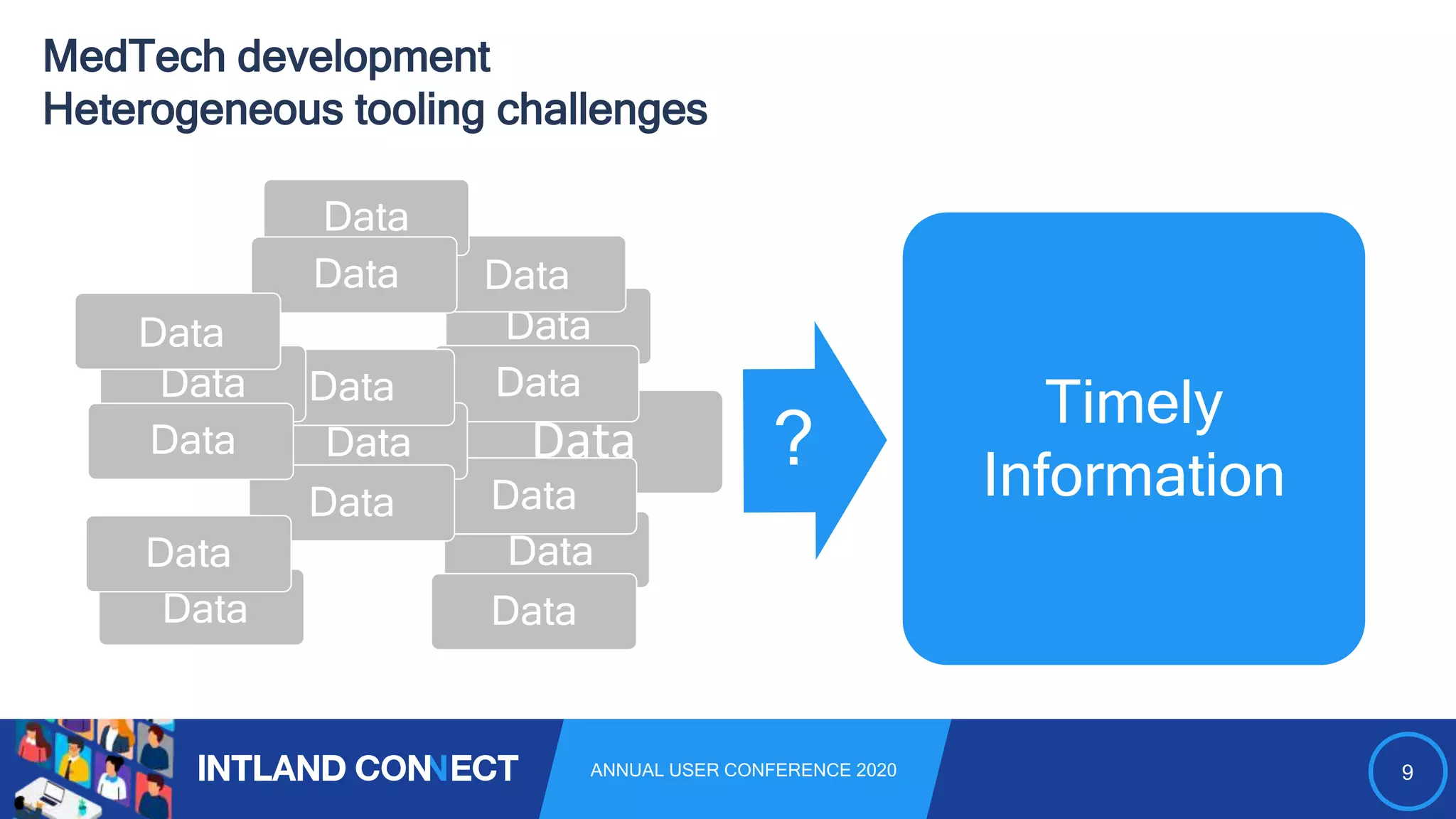
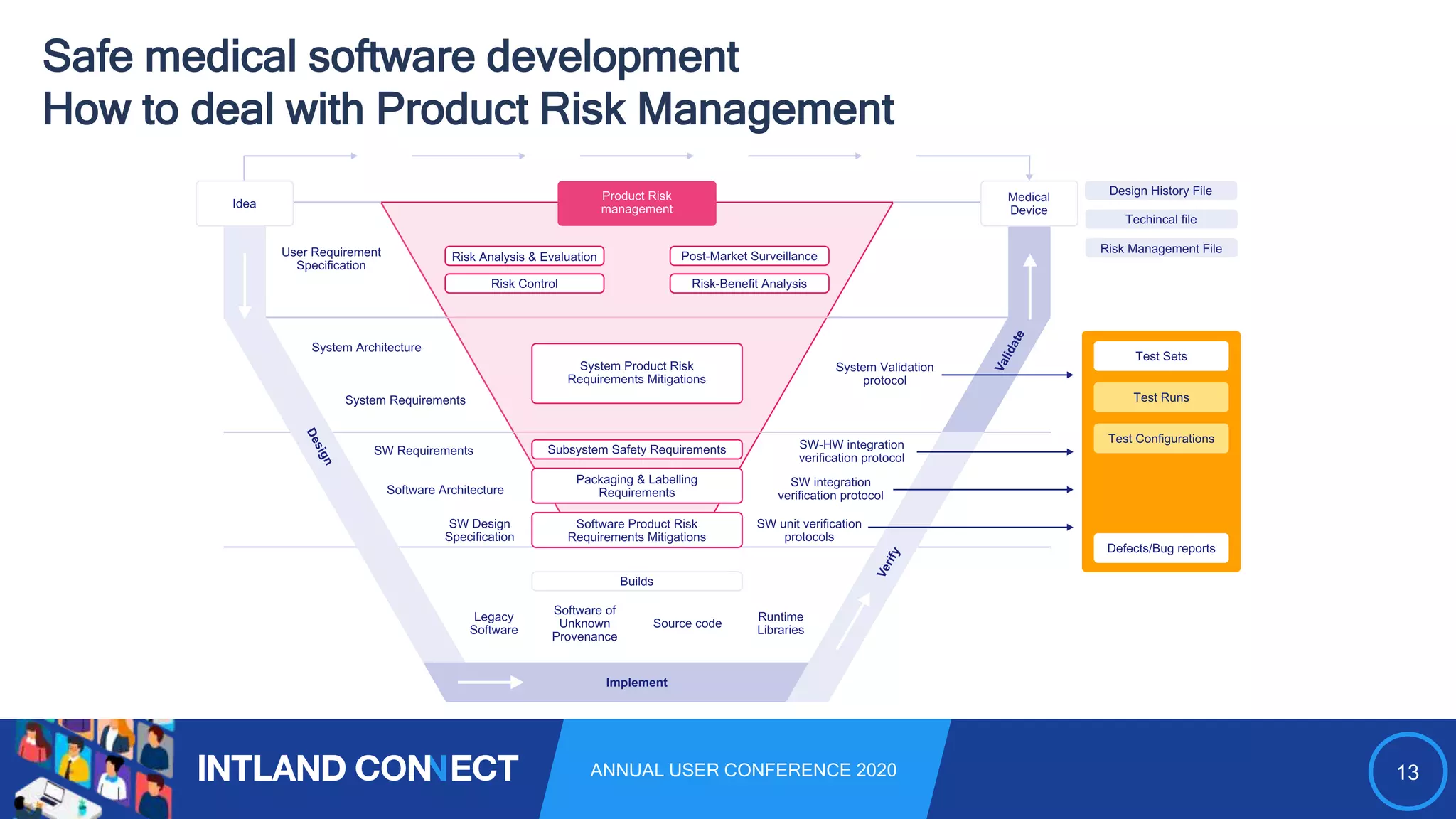
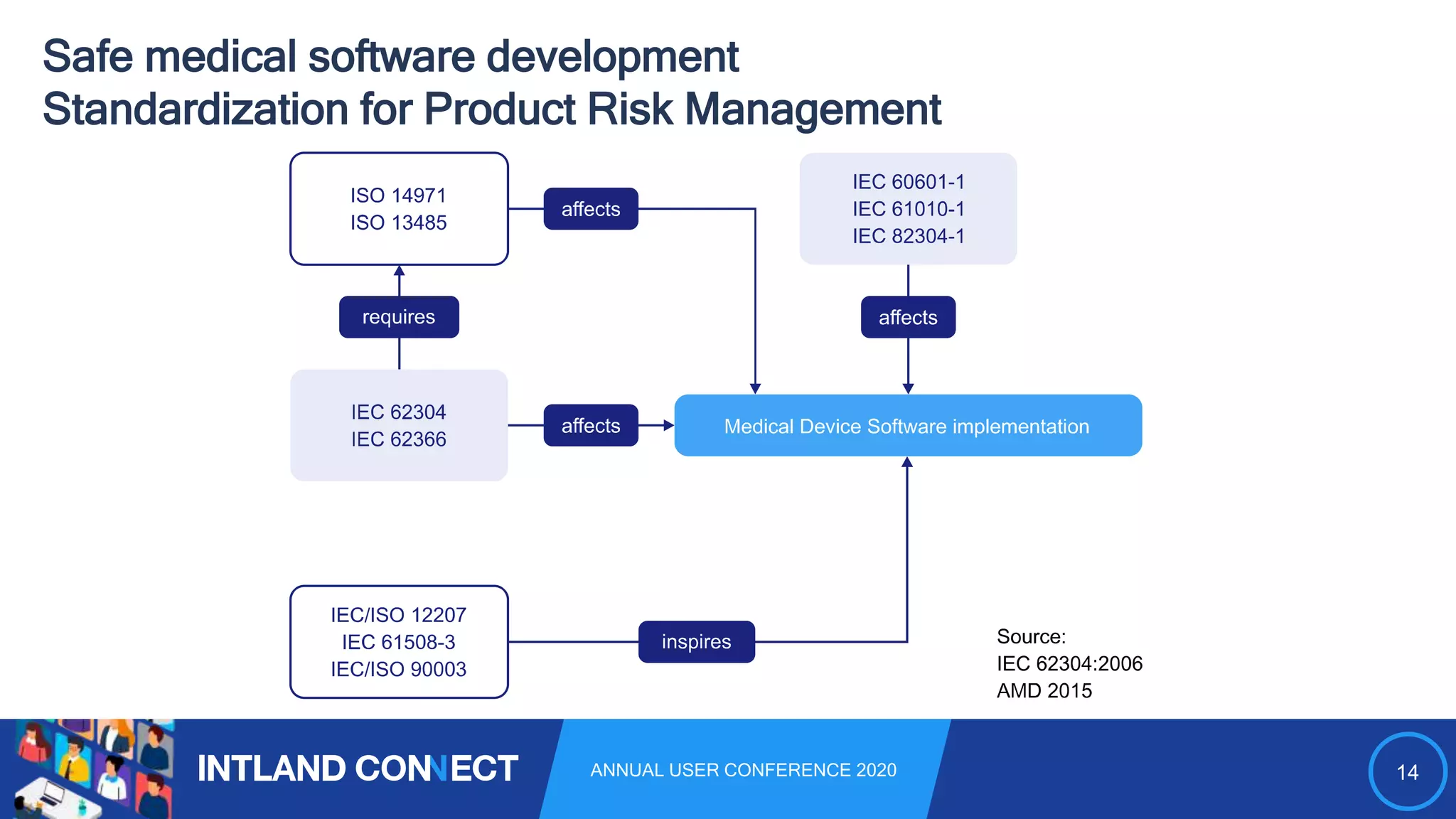
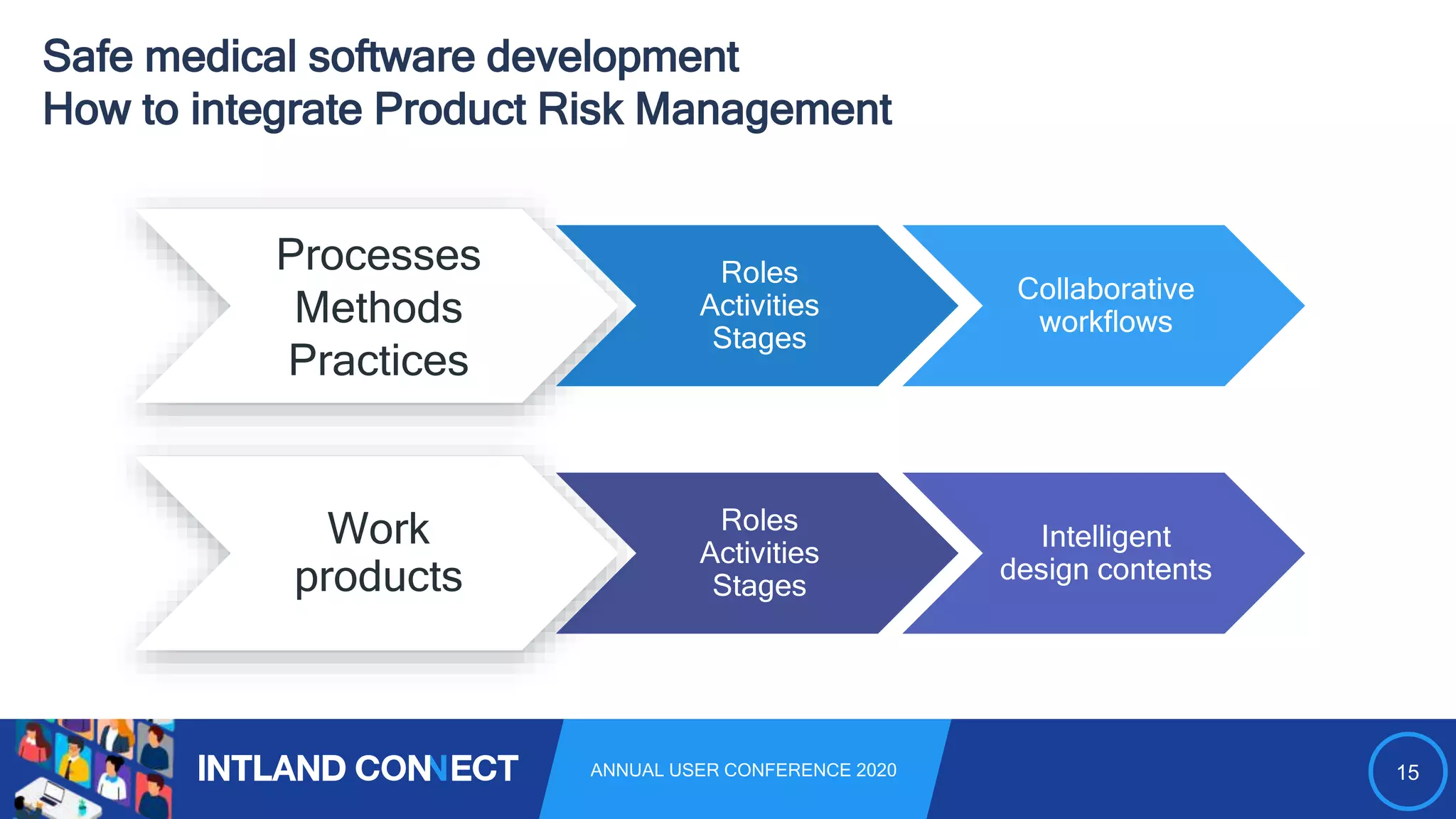
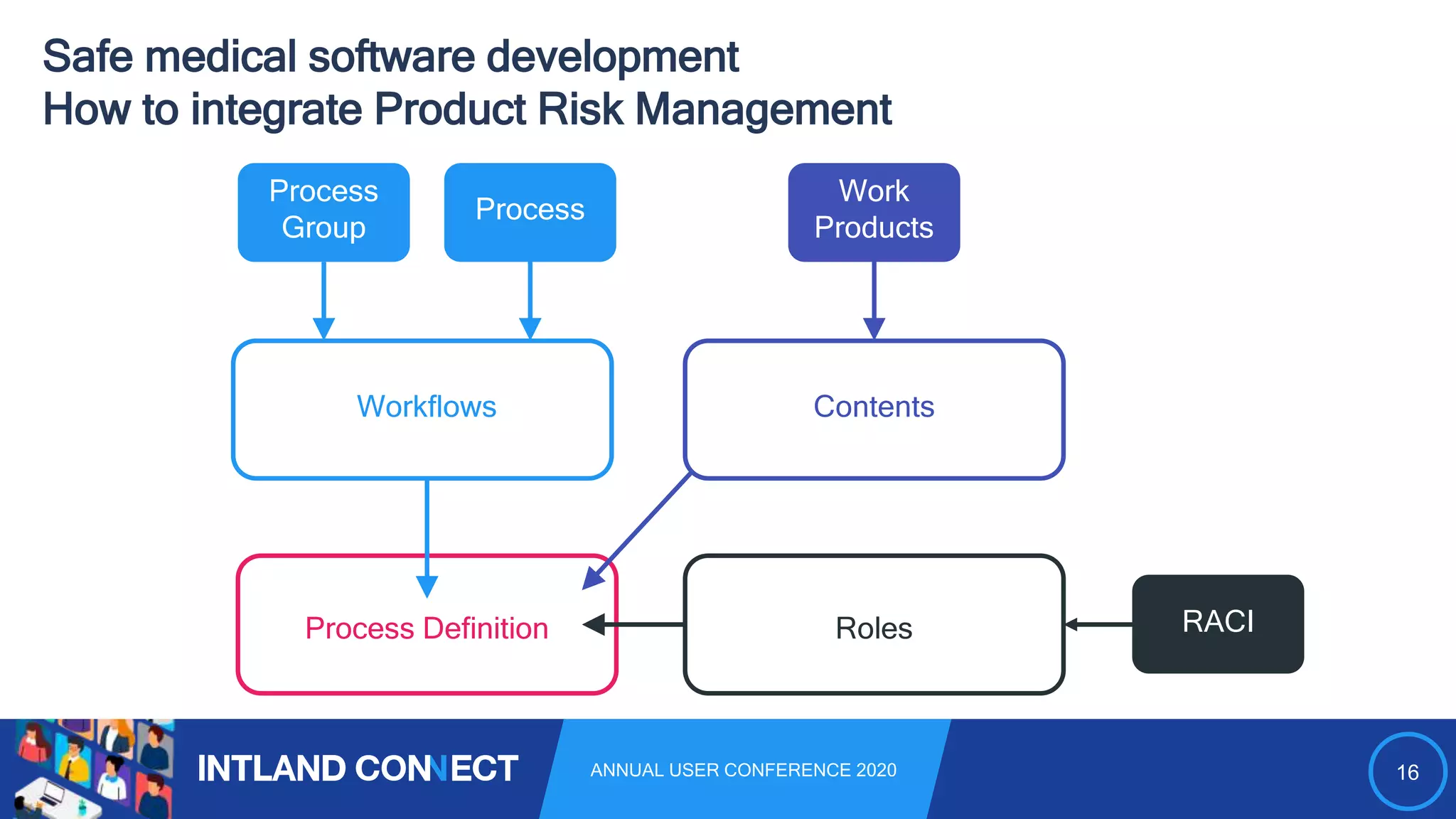
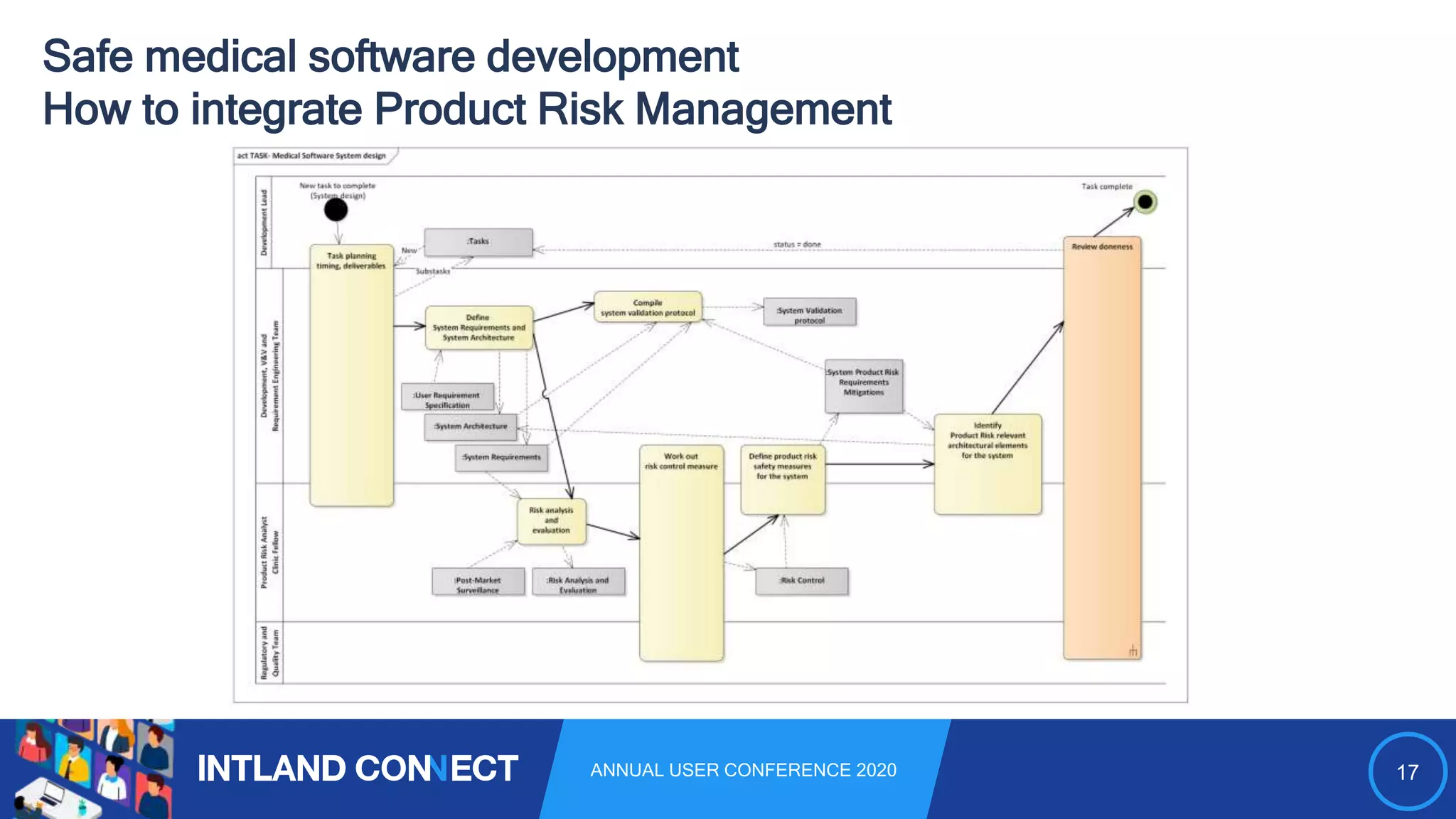
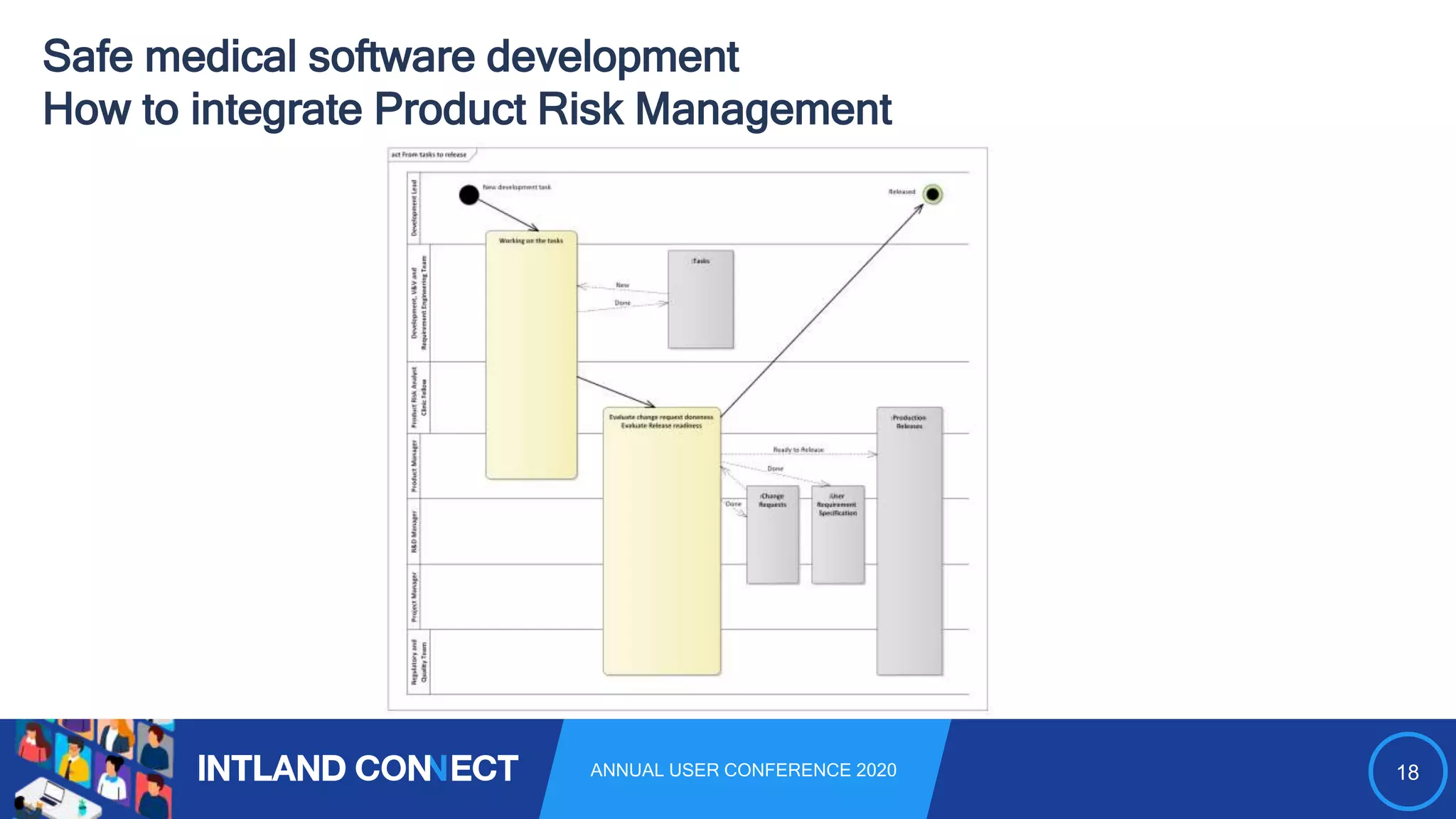
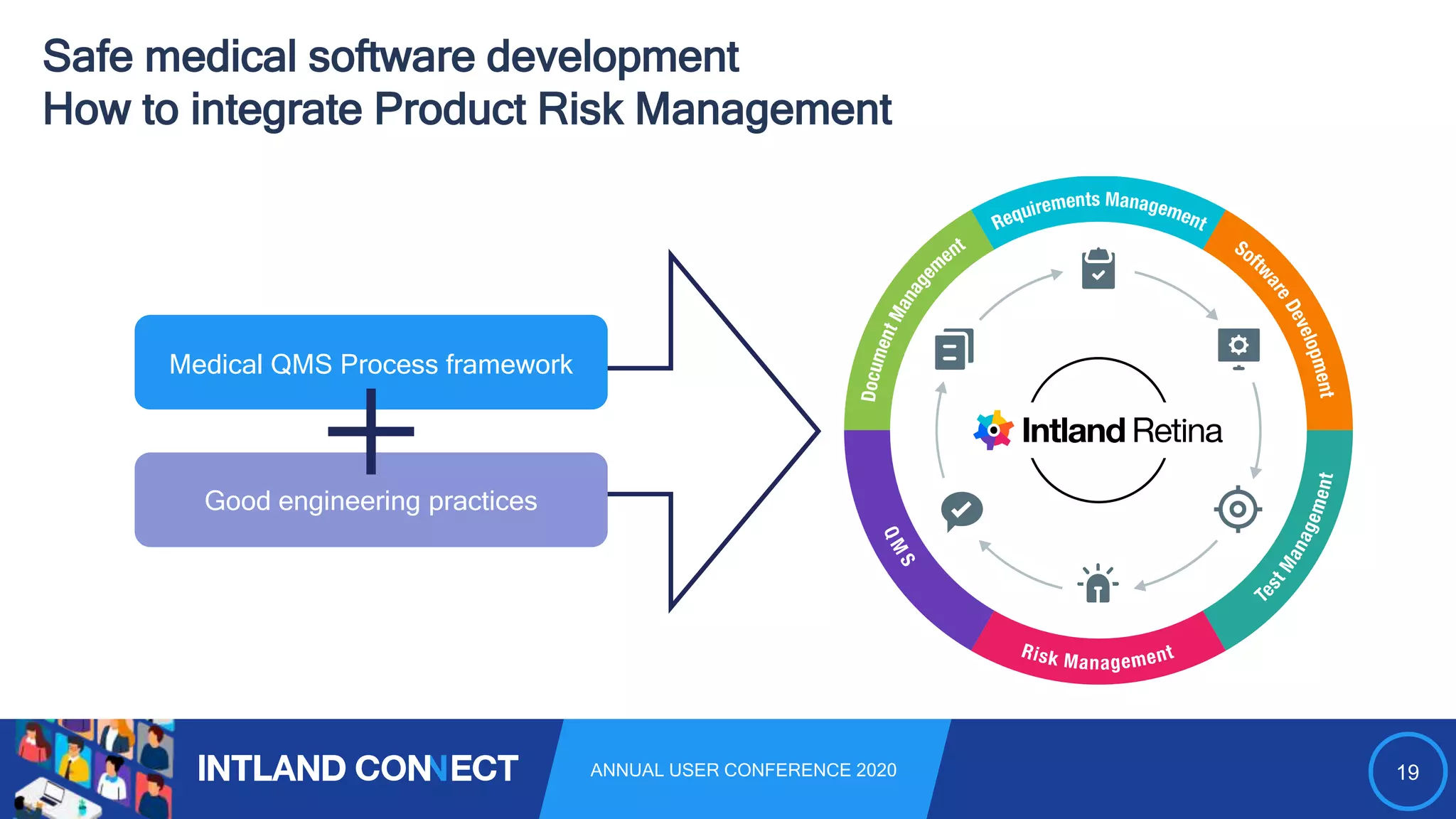
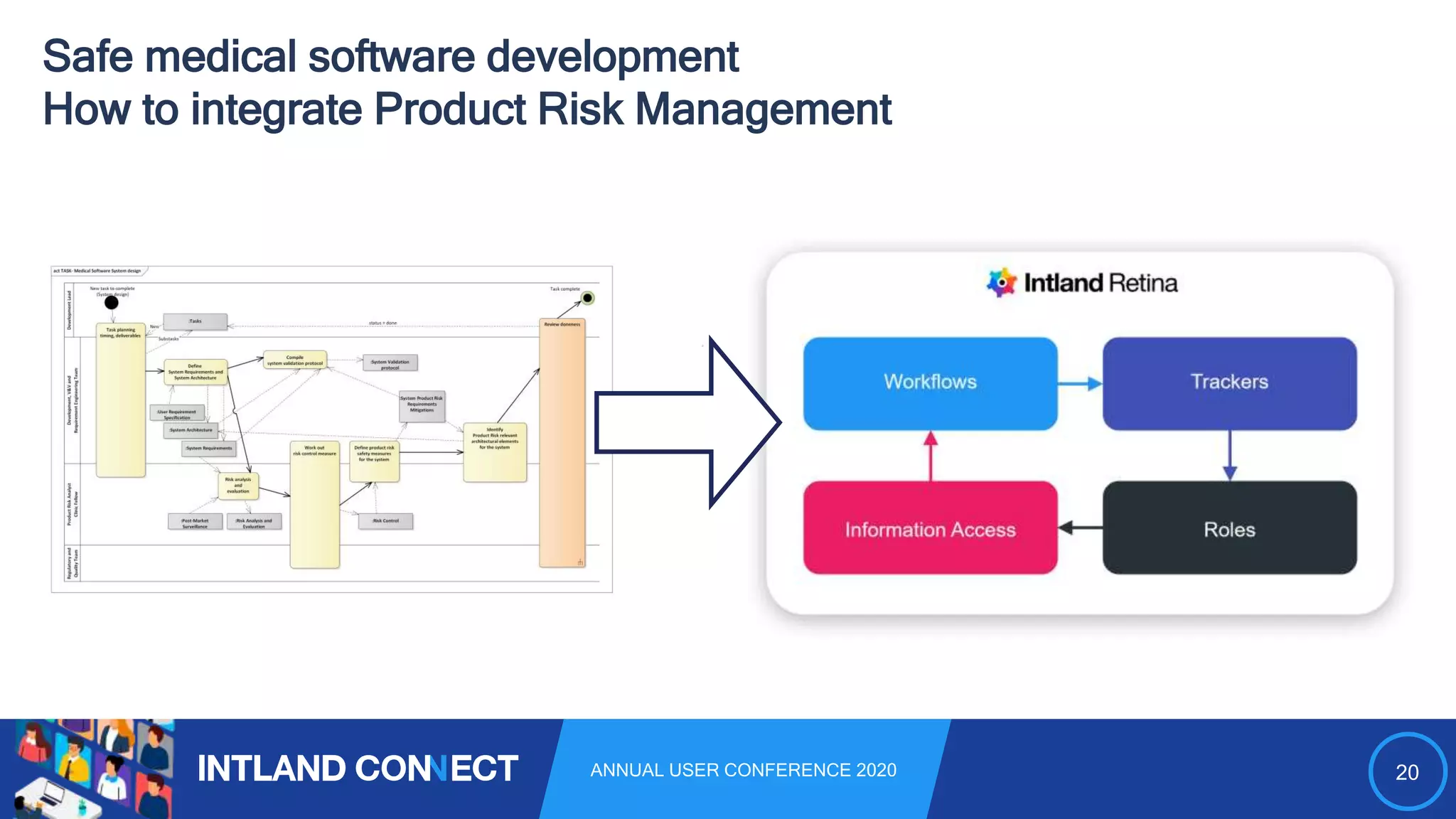
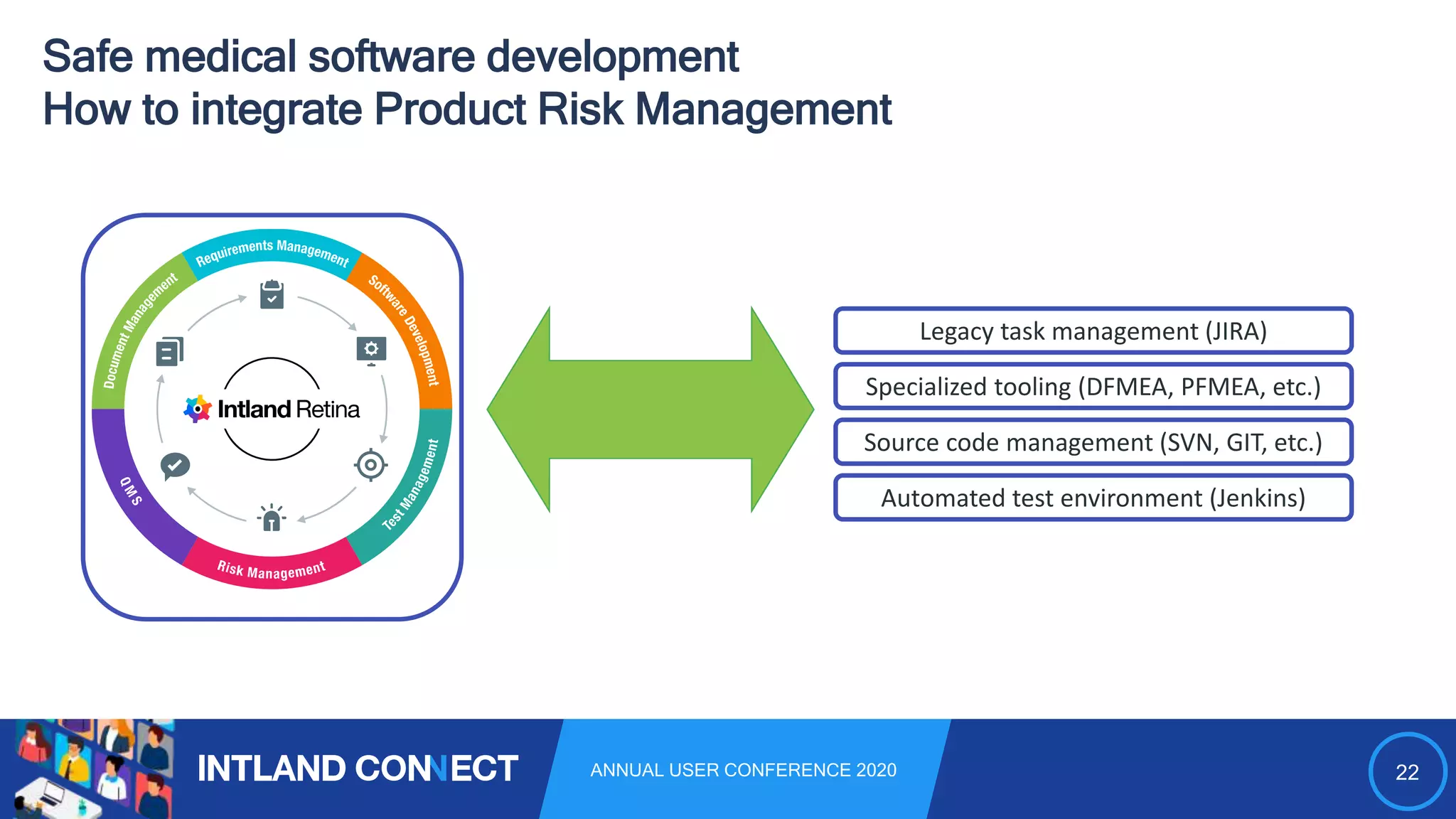
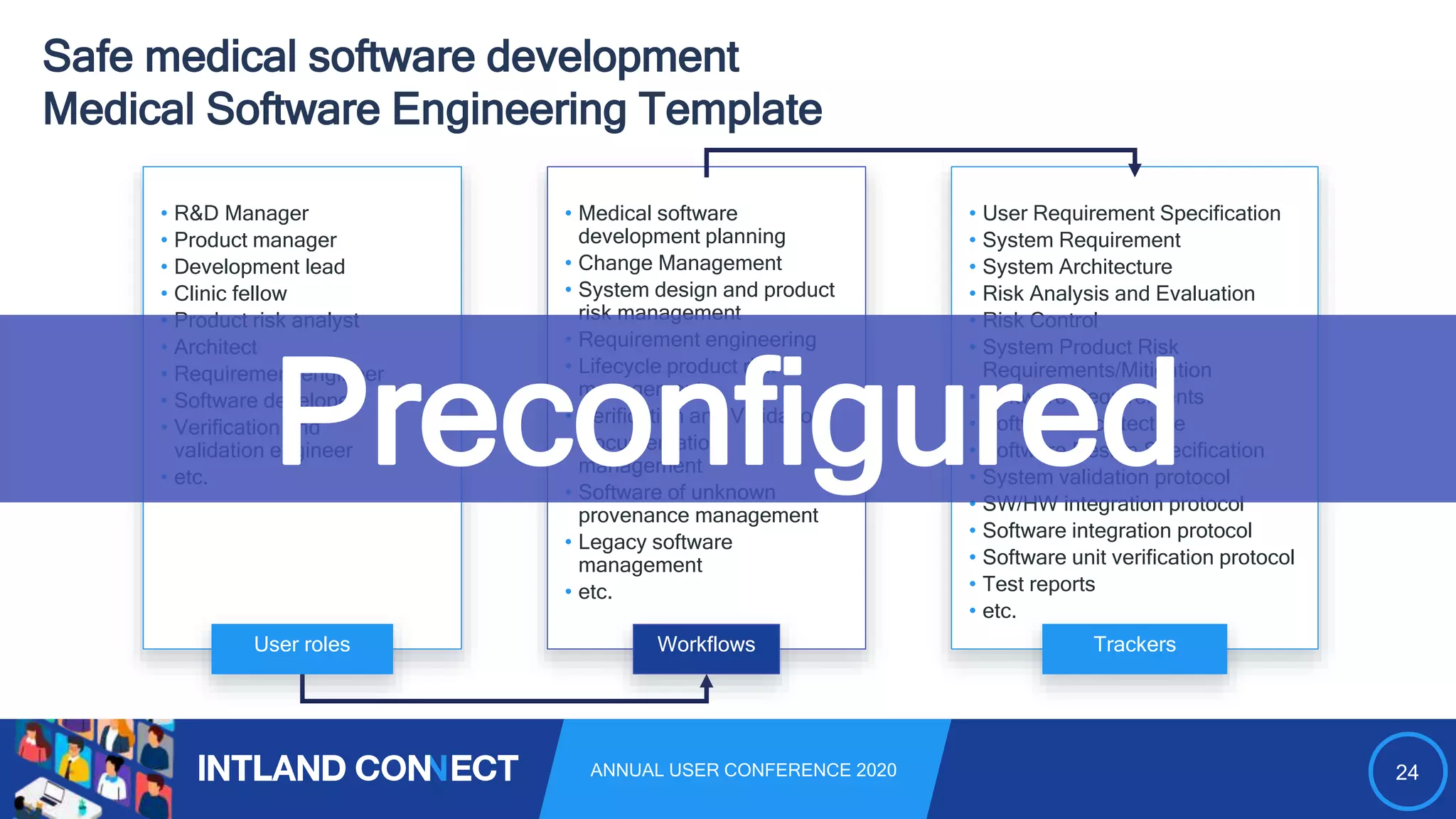
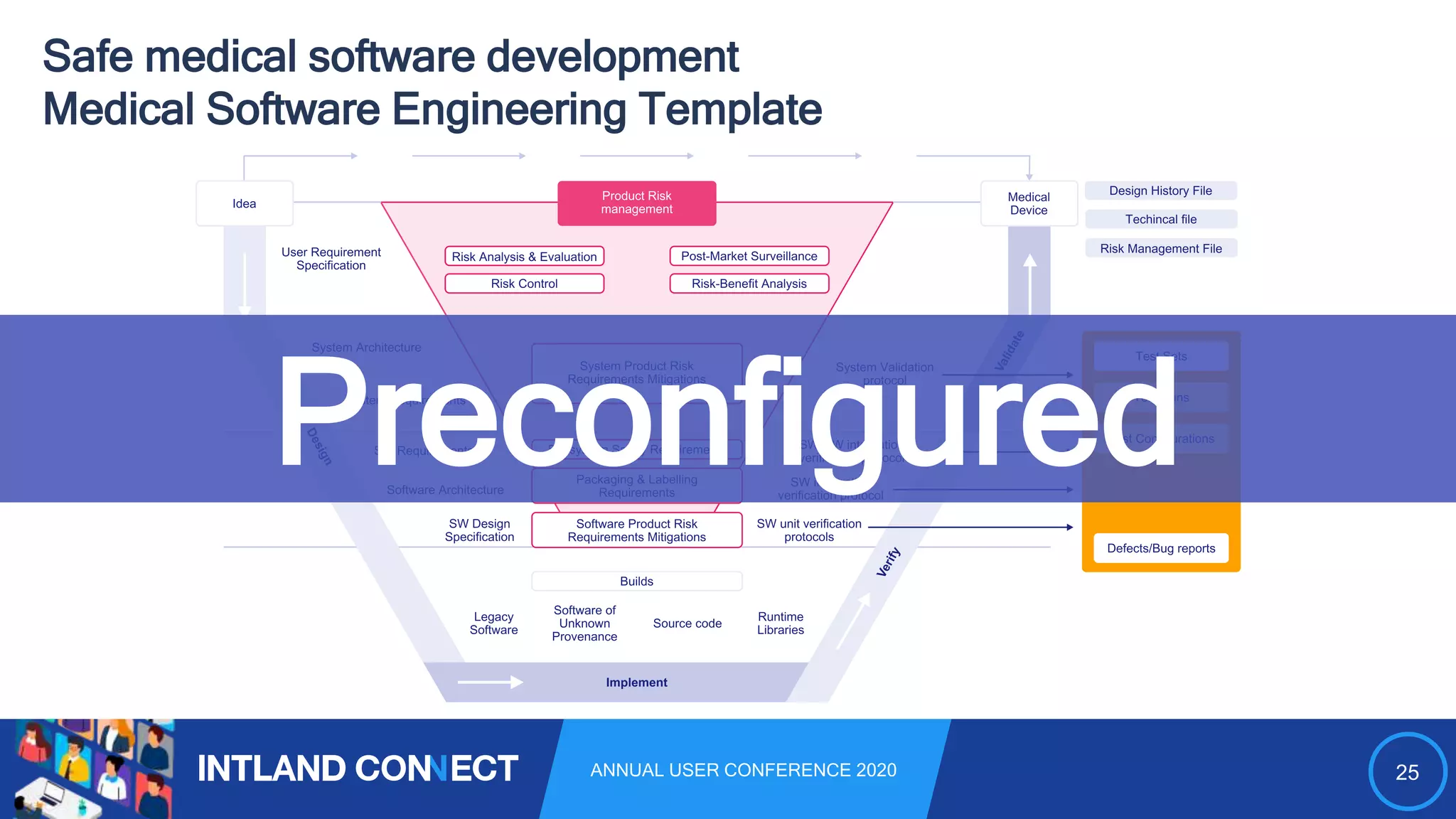
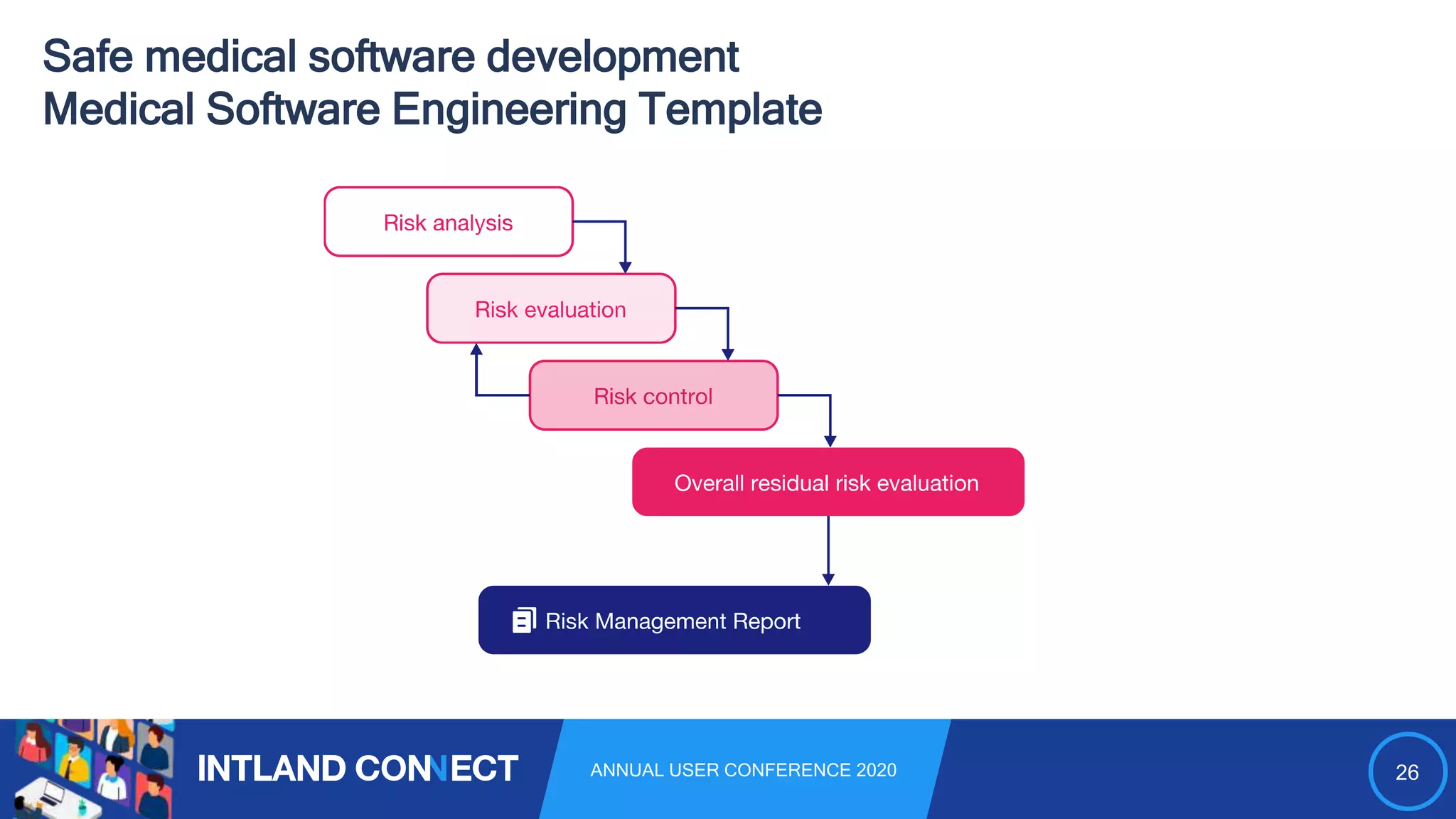
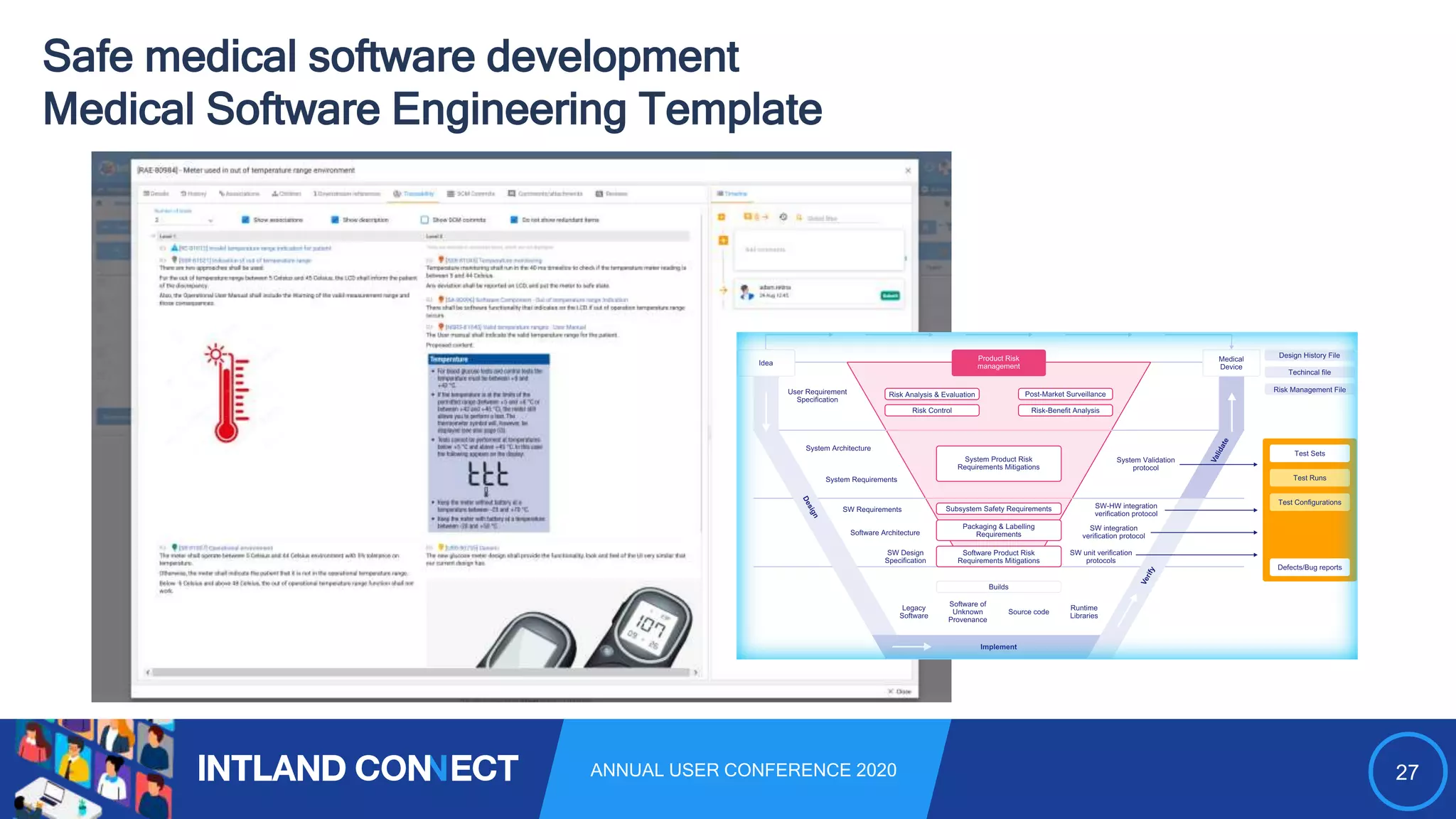
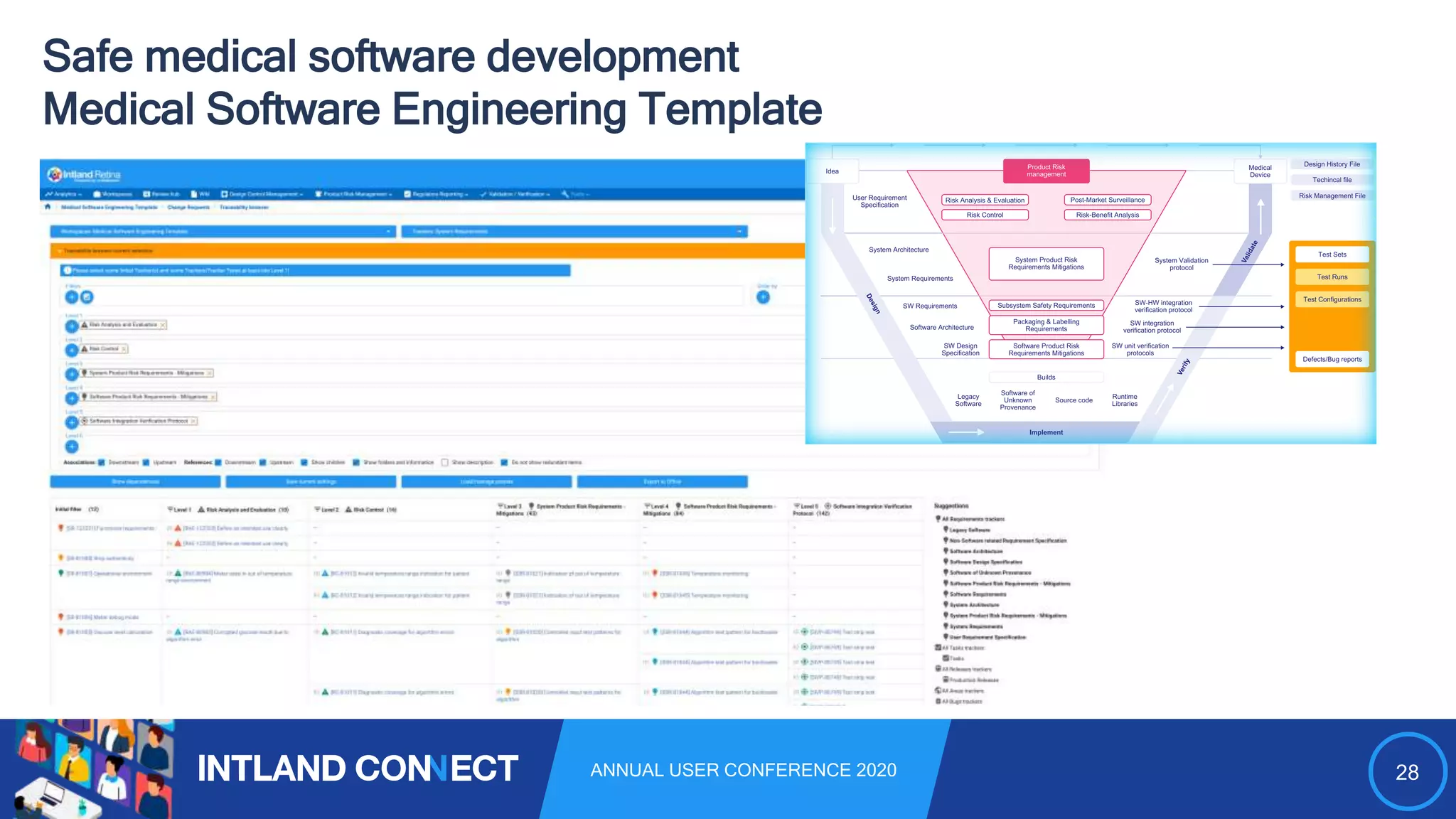
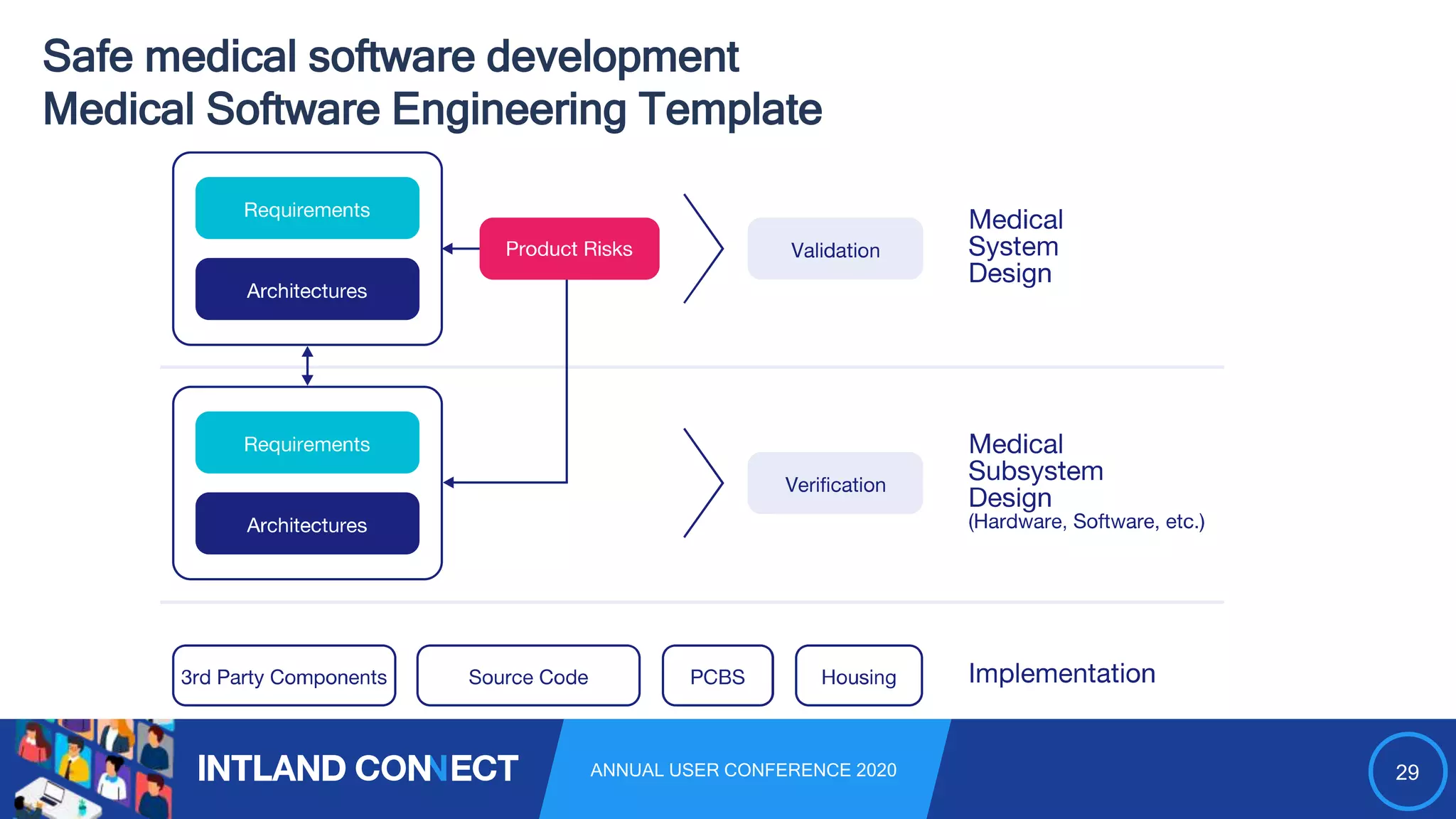
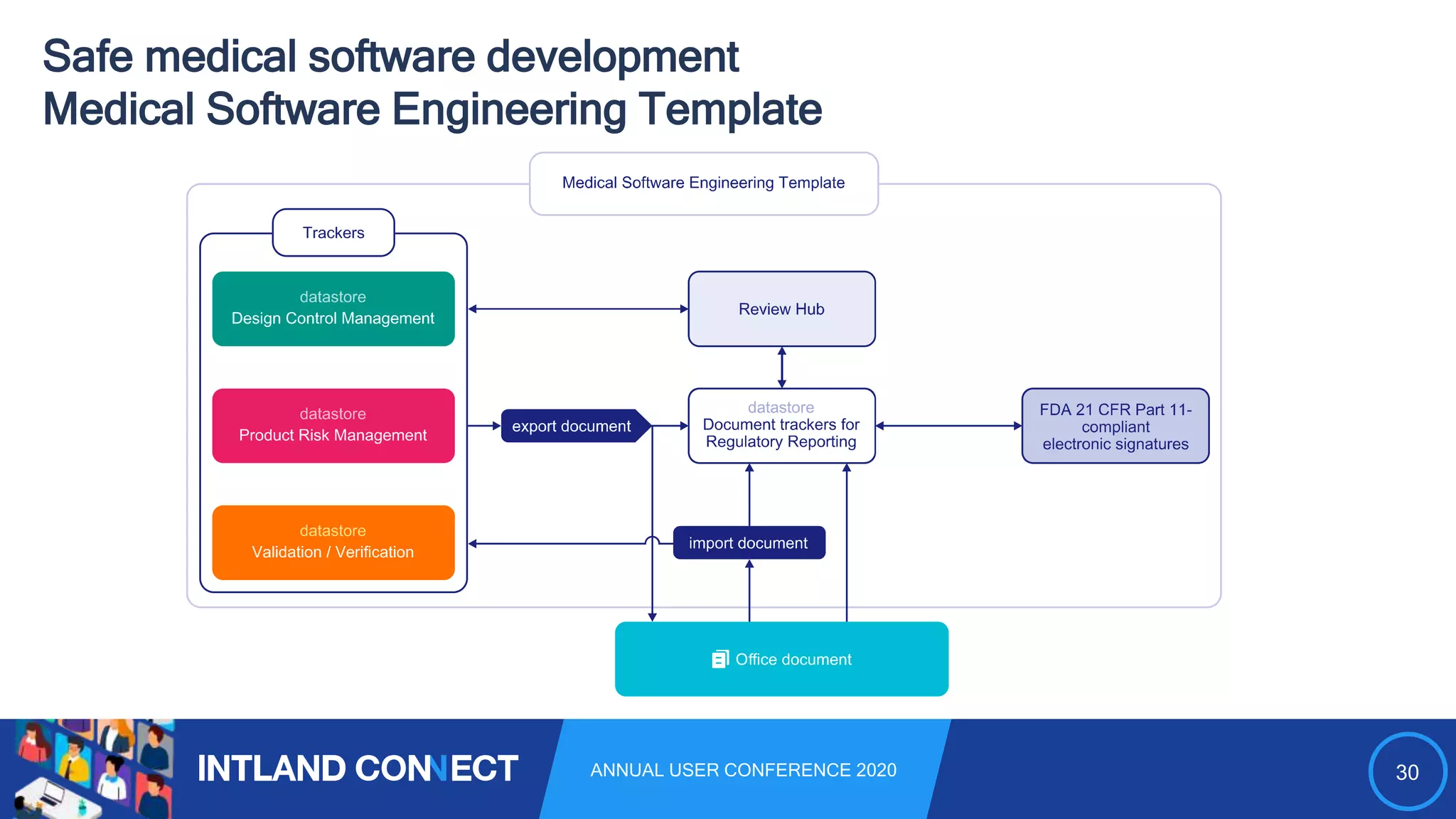
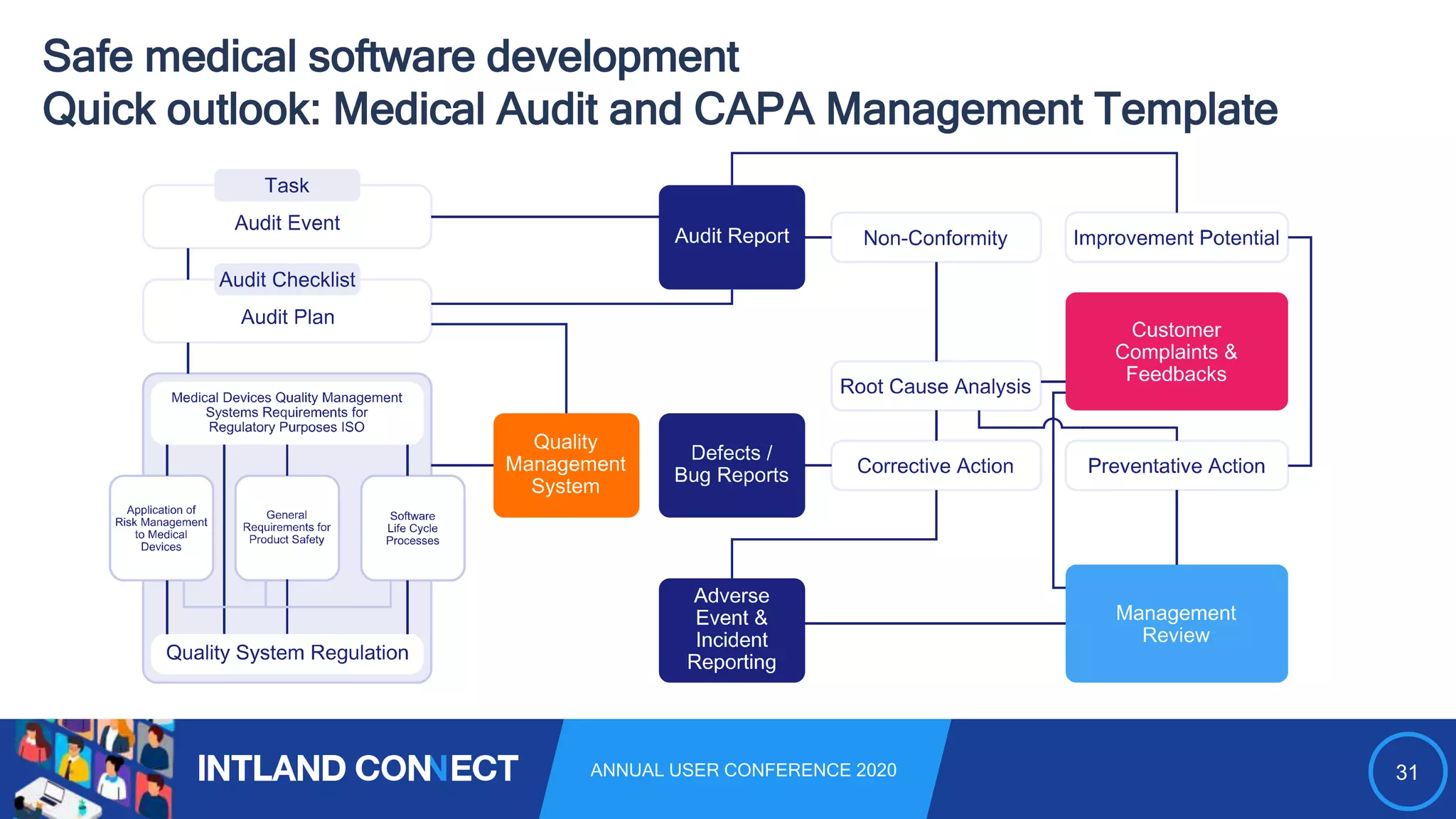
The document discusses approaches for safe medical software development, emphasizing the integration of product risk management into various processes within the medical device development lifecycle. It outlines challenges with heterogeneous tooling and the need for effective process control and transparency in design. Additionally, it introduces Intland Software's validated process templates for customizing medical software engineering practices.