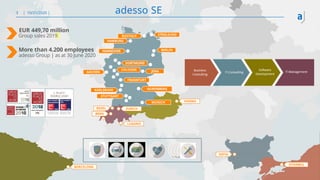
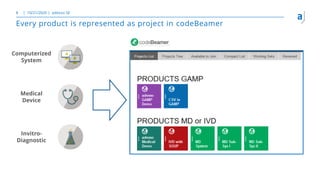
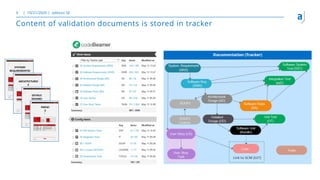
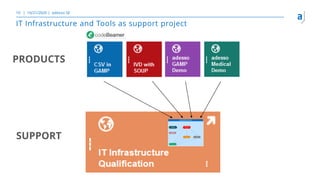
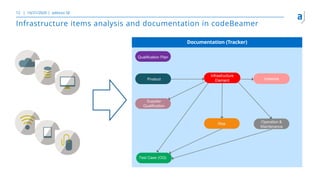
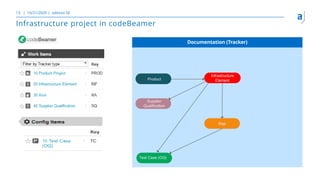
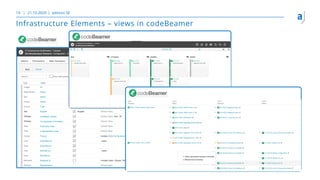
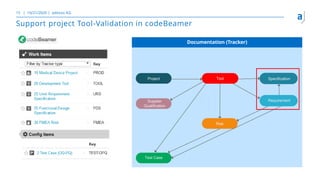
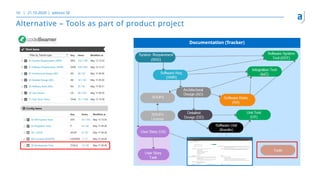
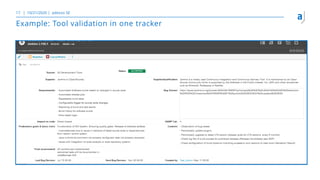
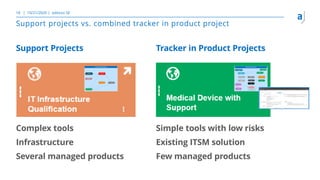
The document outlines the principles of tool validation and infrastructure qualification using codebeamer ALM at the Intland Annual User Conference 2020. It discusses compliance in software development, configuration management, and project learnings within regulated environments, particularly in medical software development. Key considerations include risk assessment, document tracking, and recommendations for effective tool validation.