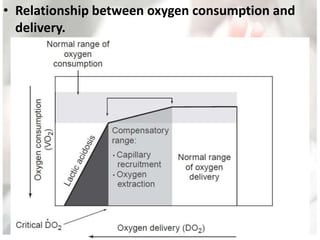
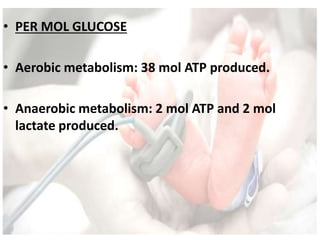
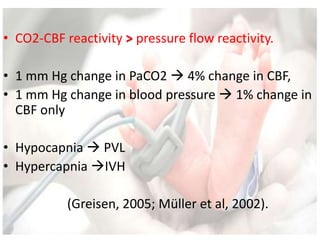
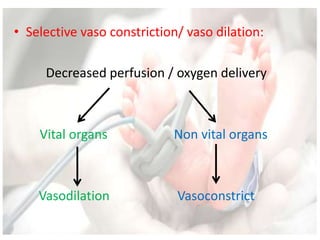
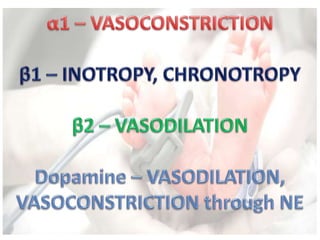
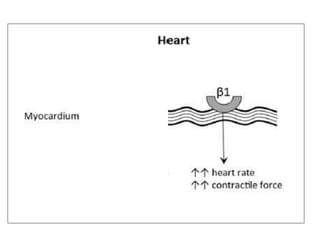
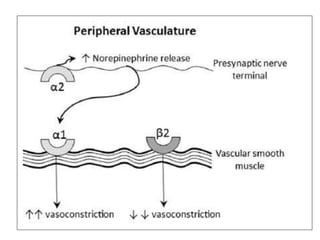
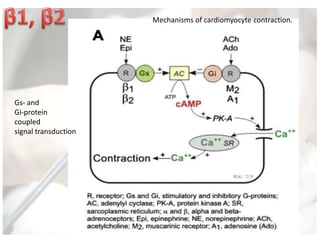
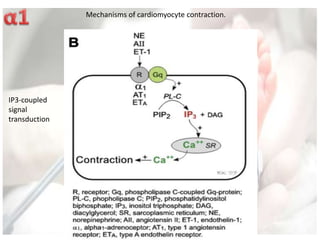
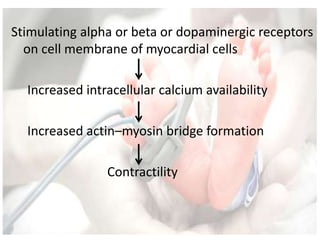
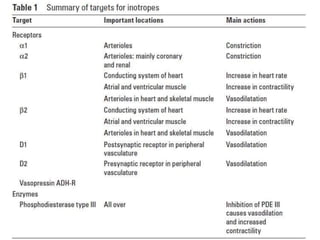
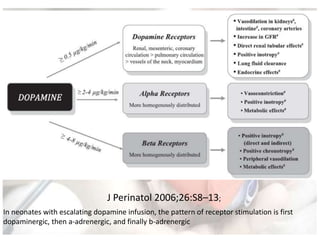
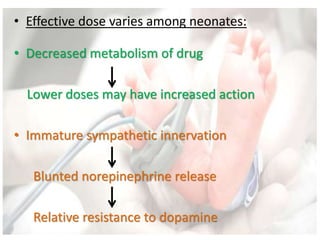
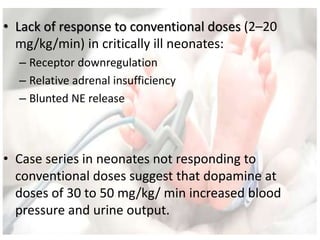
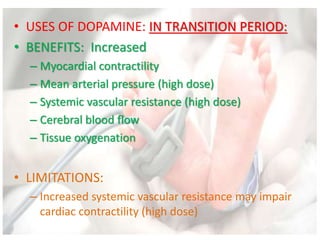
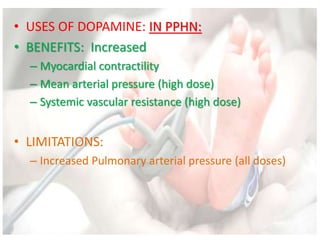
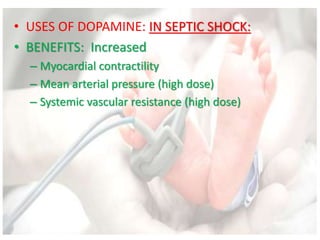
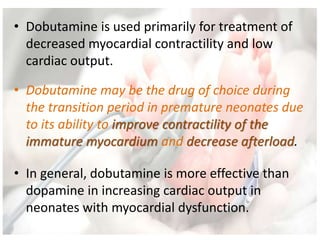
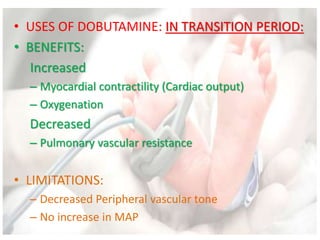
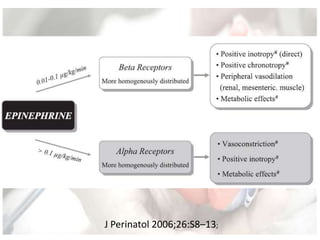
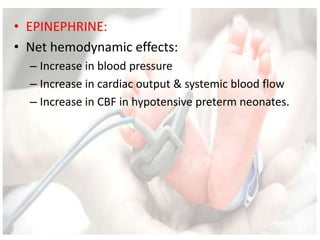
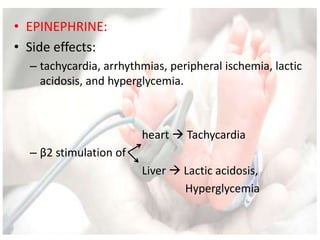
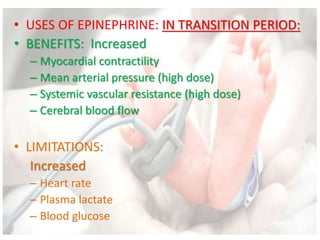
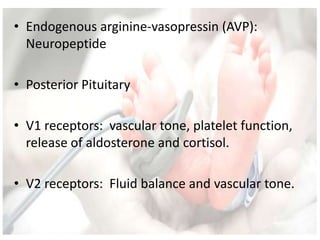
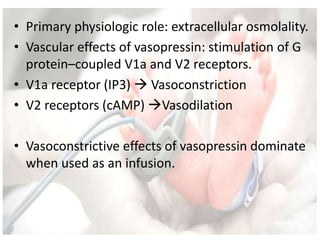
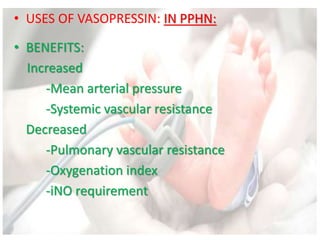
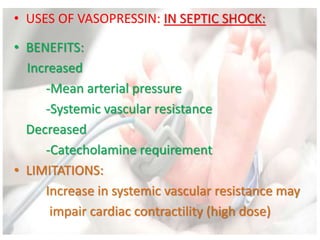
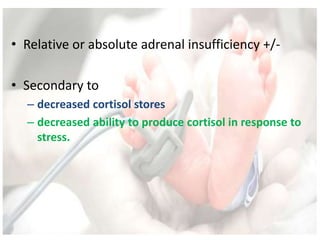
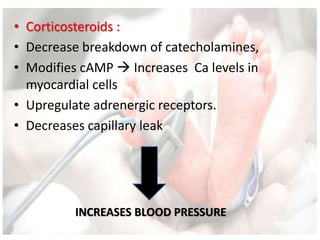
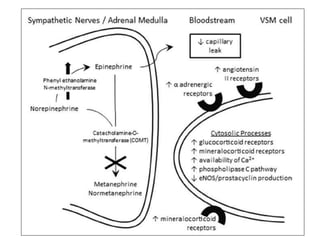
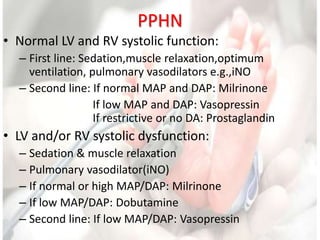
This document discusses neonatal shock, including its pathophysiology, terminology, history of inotropic drugs, and clinical uses of various inotropic agents. It covers topics such as the unique features of the preterm cardiovascular system, oxygen delivery principles, shock etiologies like hypovolemia and myocardial dysfunction, and the mechanisms and receptors targeted by drugs like dopamine, dobutamine, epinephrine, norepinephrine, milrinone, vasopressin, and corticosteroids. Clinical scenarios where different agents may be beneficial or have limitations are also summarized.







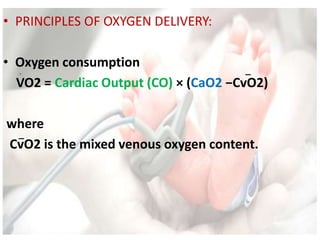
![• PRINCIPLES OF OXYGEN DELIVERY:
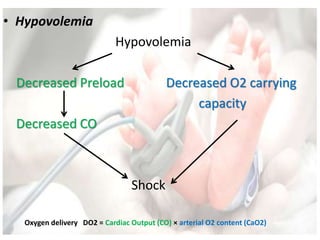
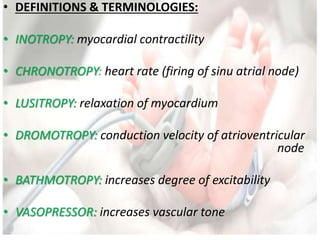
• Oxygen delivery
DO2 = Cardiac Output (CO) × arterial O2 content (CaO2)
where
CO = HR × stroke volume (SV)
&
CaO2 = [1.34 × Hb × SaO2] + [0.003 × PaO2]](https://image.slidesharecdn.com/inotropesinneonatesdrpadmesh-161021142321/85/Shock-Inotropes-in-Neonates-Dr-Padmesh-Neonatology-11-320.jpg)
![• PRINCIPLES OF OXYGEN DELIVERY:
• Oxygen delivery
DO2 = Cardiac Output (CO) × arterial O2 content (CaO2)
where
CO = HR × stroke volume (SV)
&
CaO2 = [1.34 × Hb × SaO2] + [0.003 × PaO2]
1.Preload
2.Afterload
3.Contractility](https://image.slidesharecdn.com/inotropesinneonatesdrpadmesh-161021142321/85/Shock-Inotropes-in-Neonates-Dr-Padmesh-Neonatology-12-320.jpg)