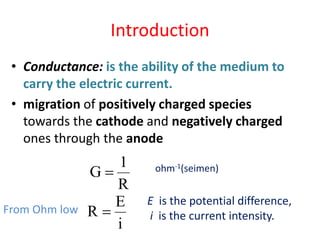
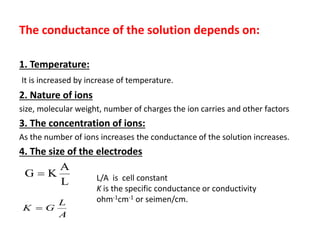
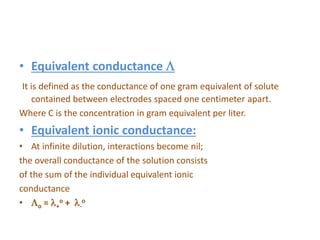
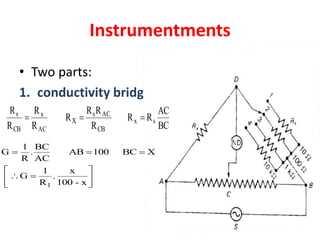
This document discusses conductometry, which is the measurement of a solution's ability to conduct electricity. Conductance depends on temperature, ion nature, concentration, and electrode size. Equivalent conductance is defined as conductance between 1 cm electrodes of 1 gram equivalent of solute per liter. At infinite dilution, equivalent ionic conductance is the sum of individual ion conductances. Conductivity is measured using a conductivity bridge and conductance cell. Conductometric titrations can be used to determine purity, physical constants, incomplete reactions, and mixtures. Curves of conductivity versus titrant volume are shown for strong acid-strong base and weak acid-strong base titrations.