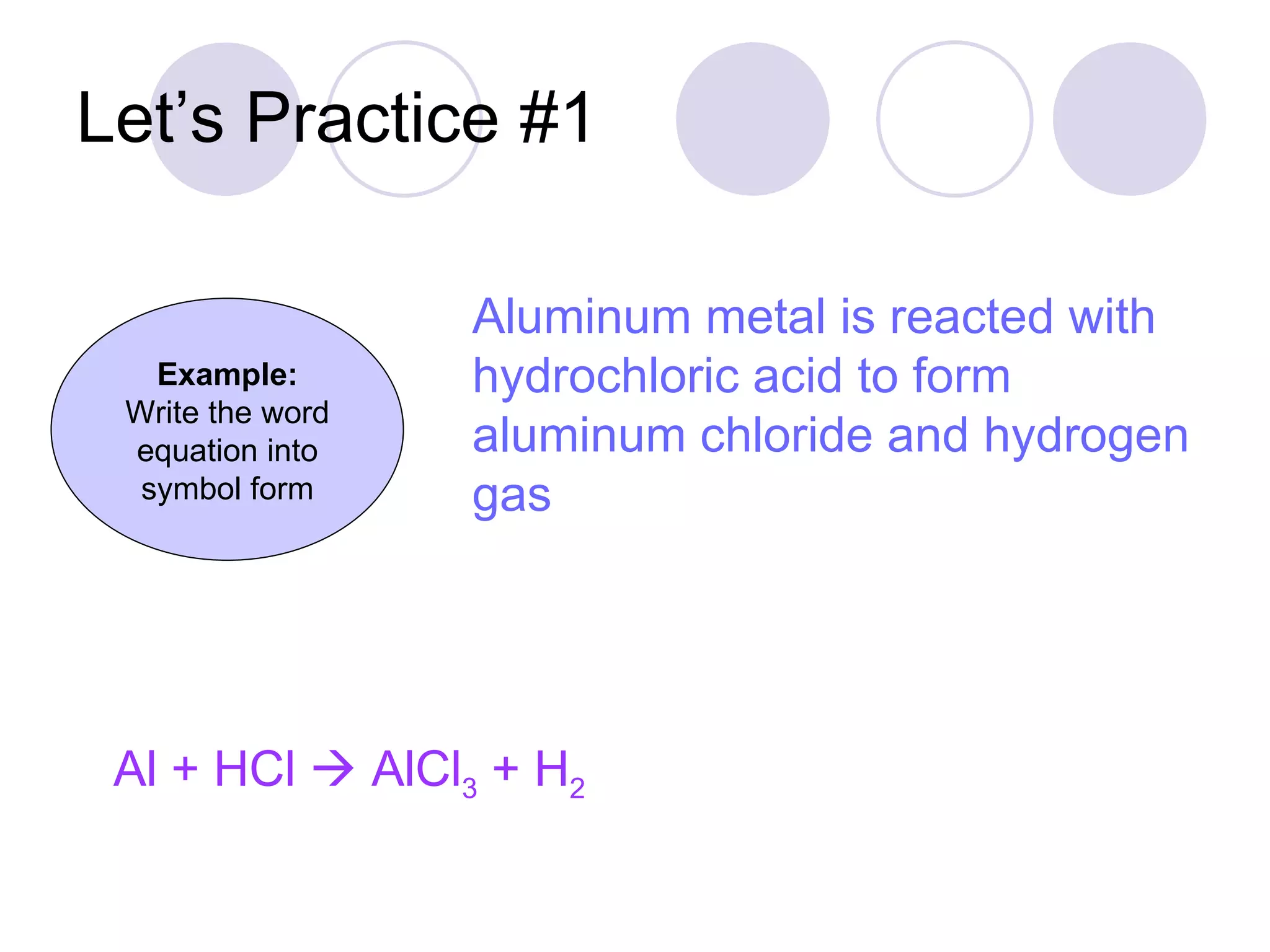
The document discusses chemical reactions and how to write chemical equations. It explains that chemical reactions involve rearranging bonds and atoms to form new compounds that are different than the starting materials. It provides examples of word and formula equations and shows the parts of a chemical equation including reactants, products, and physical states. It also instructs how to write chemical equations by converting word equations to symbolic equations using chemical formulas and reaction arrows.