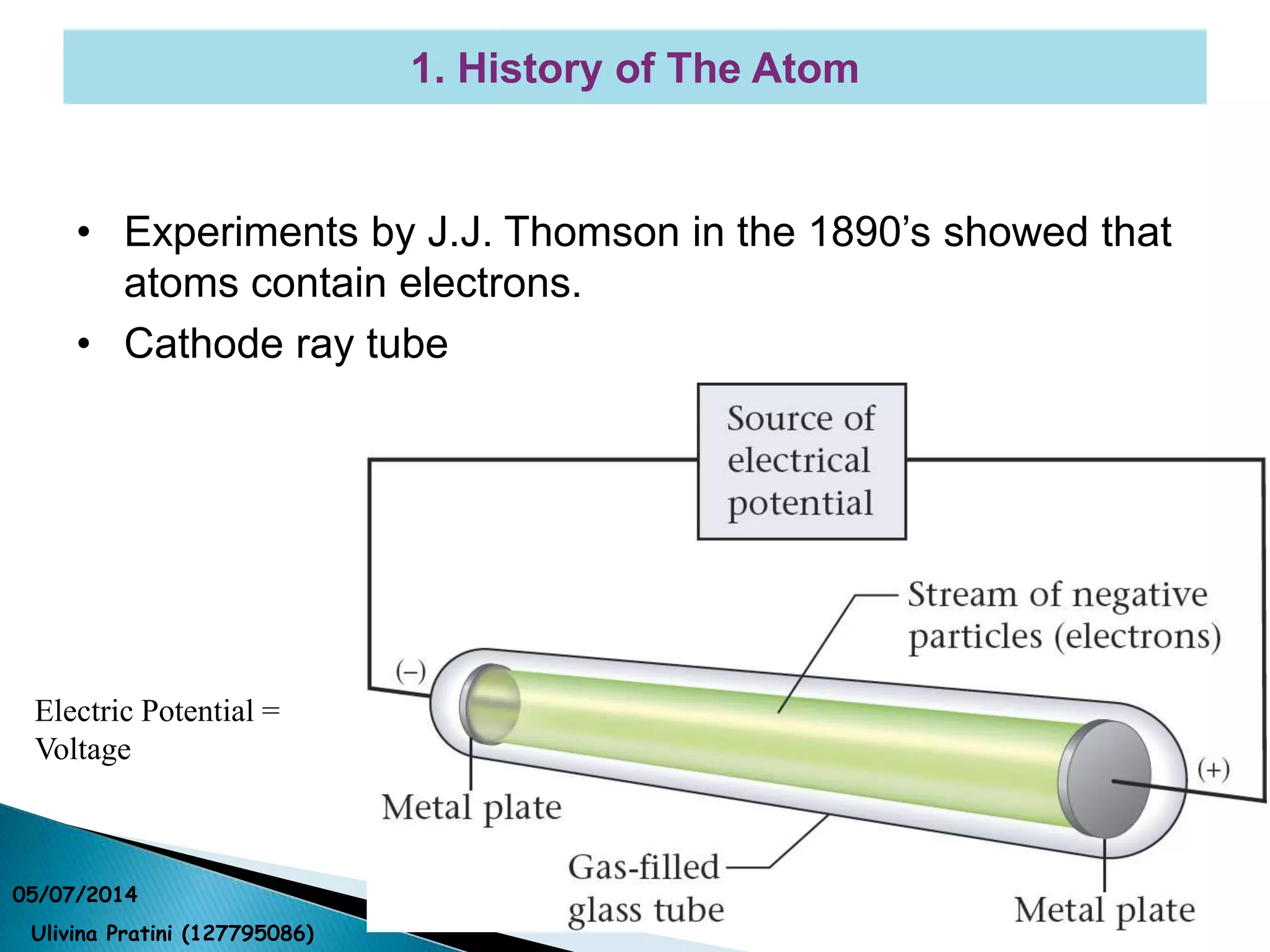
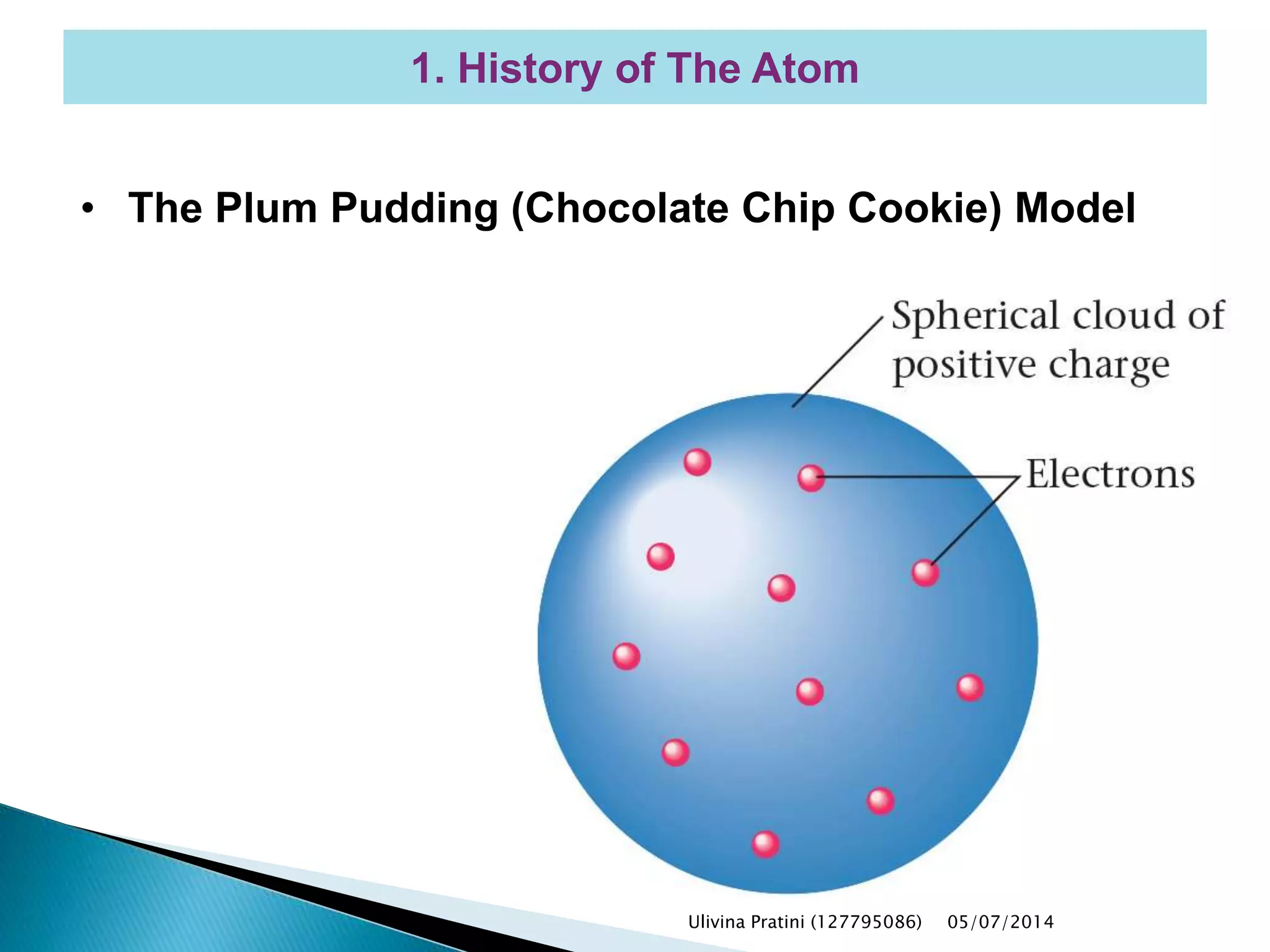
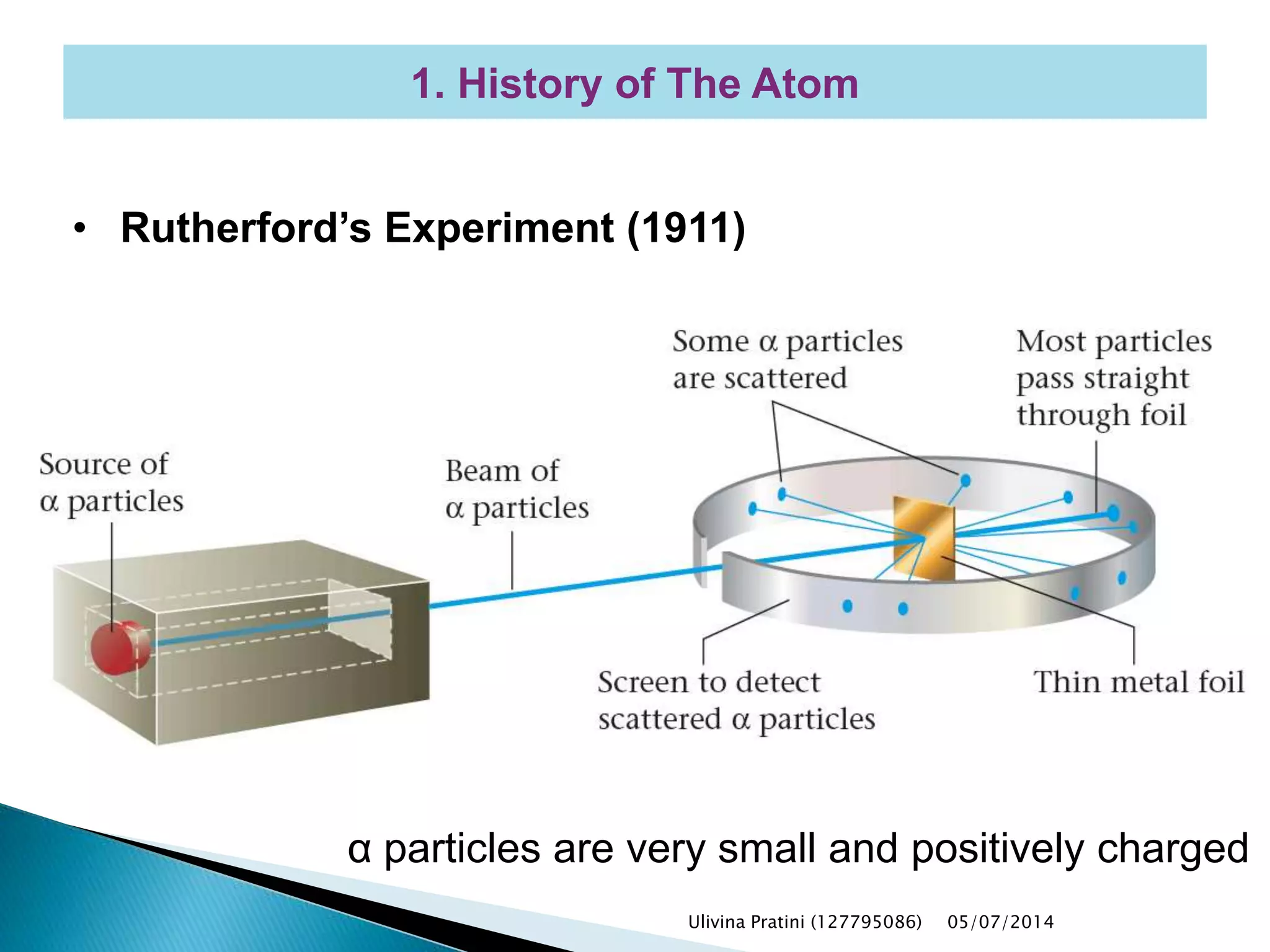
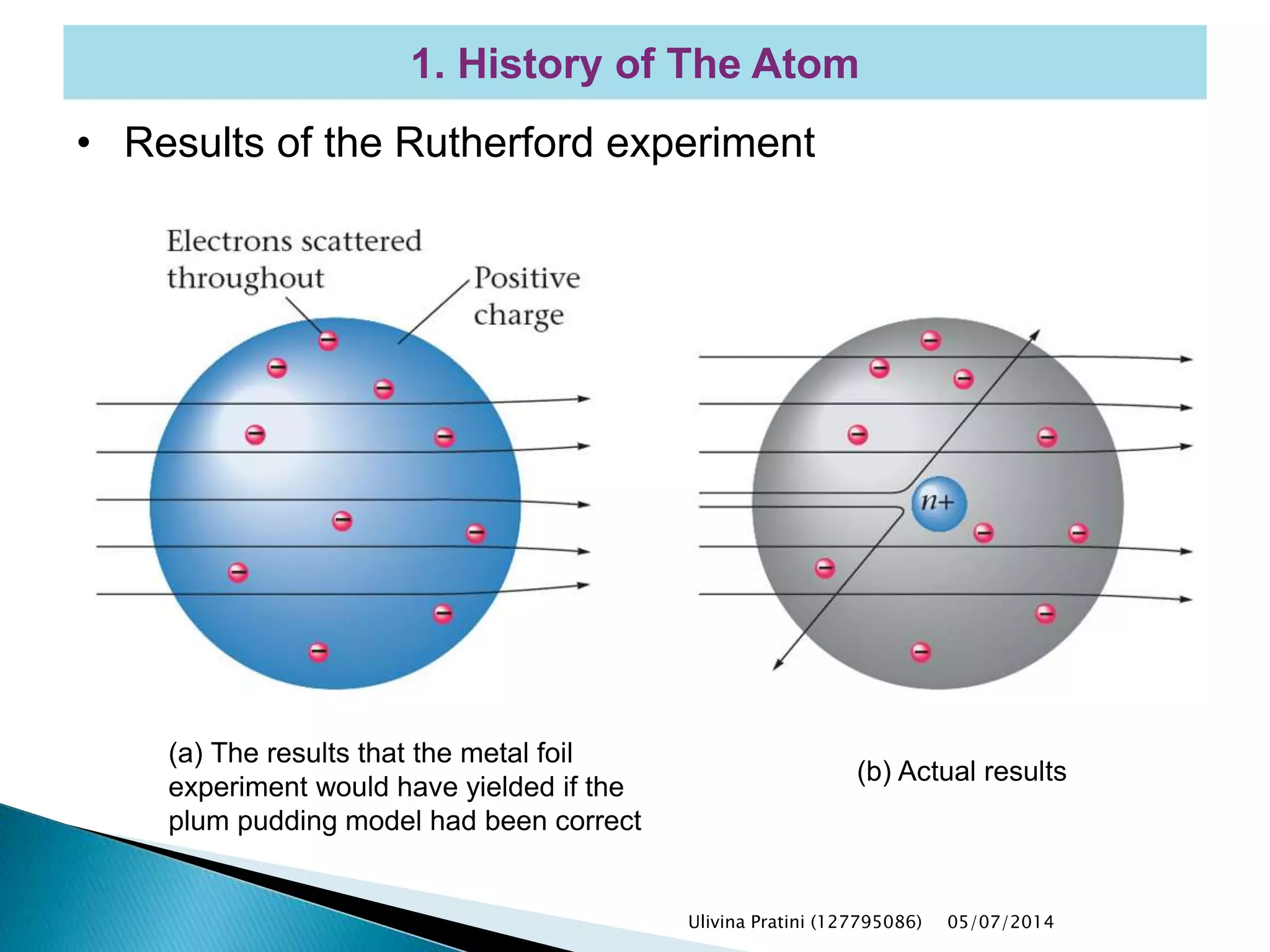
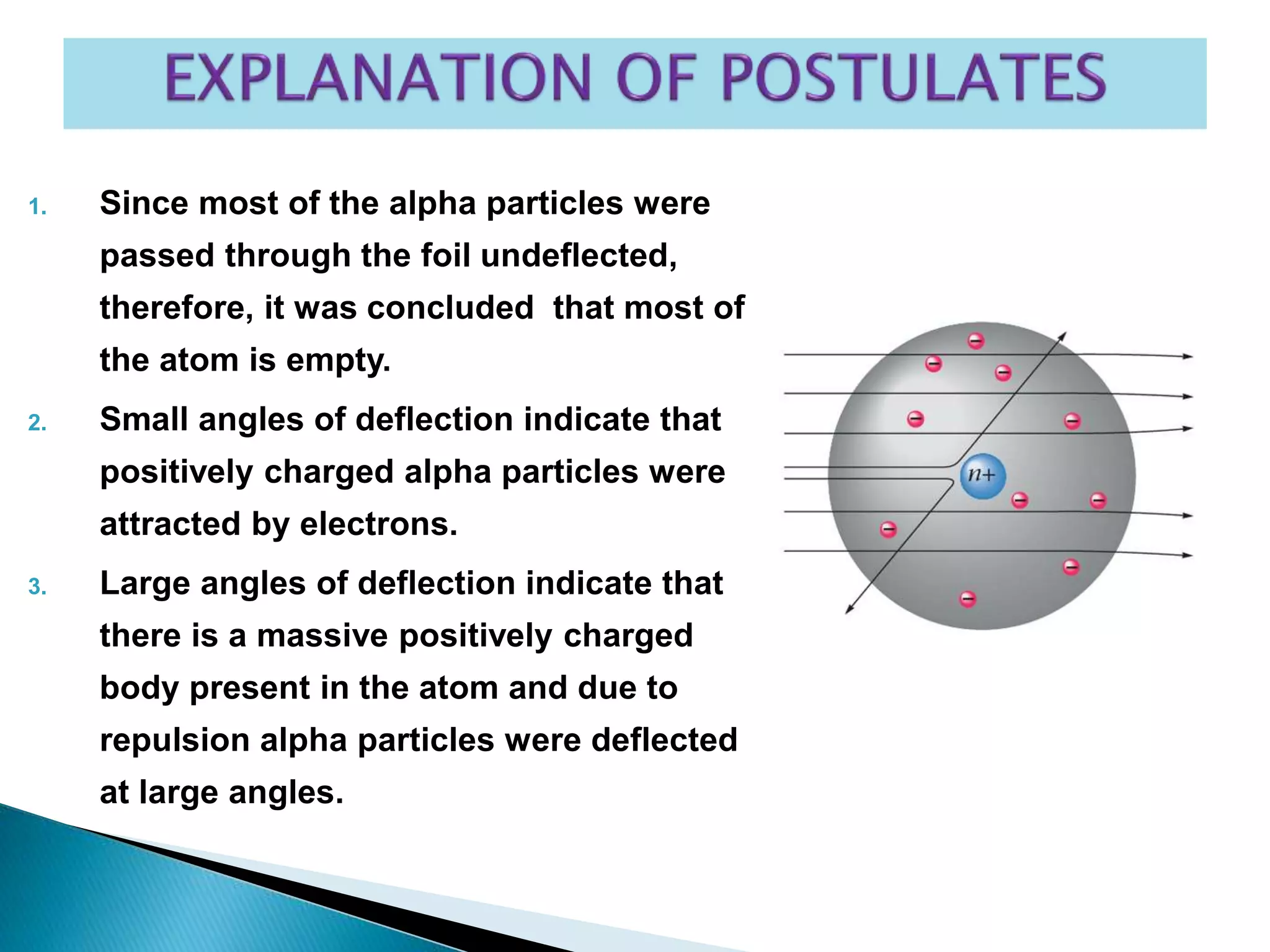
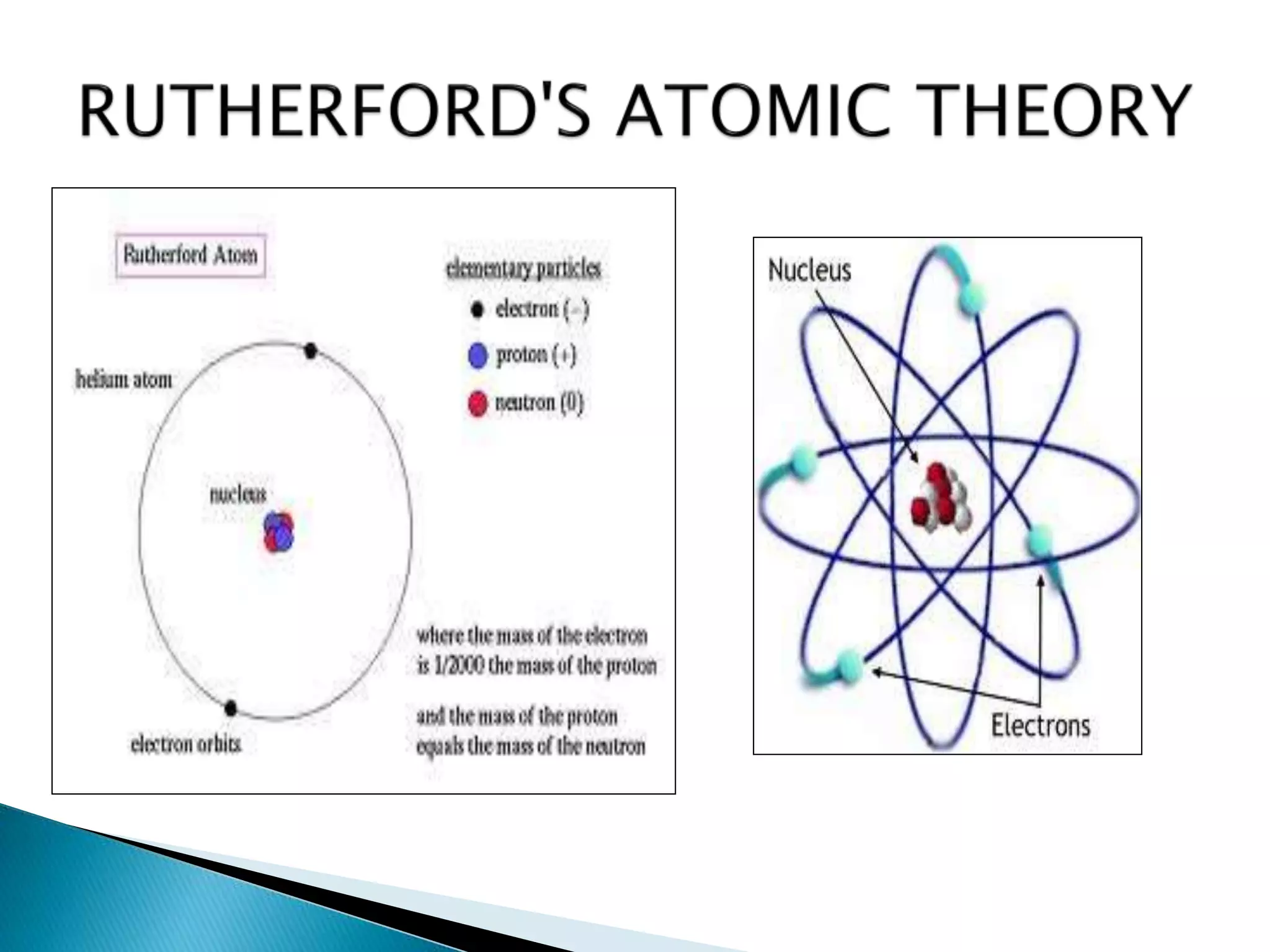
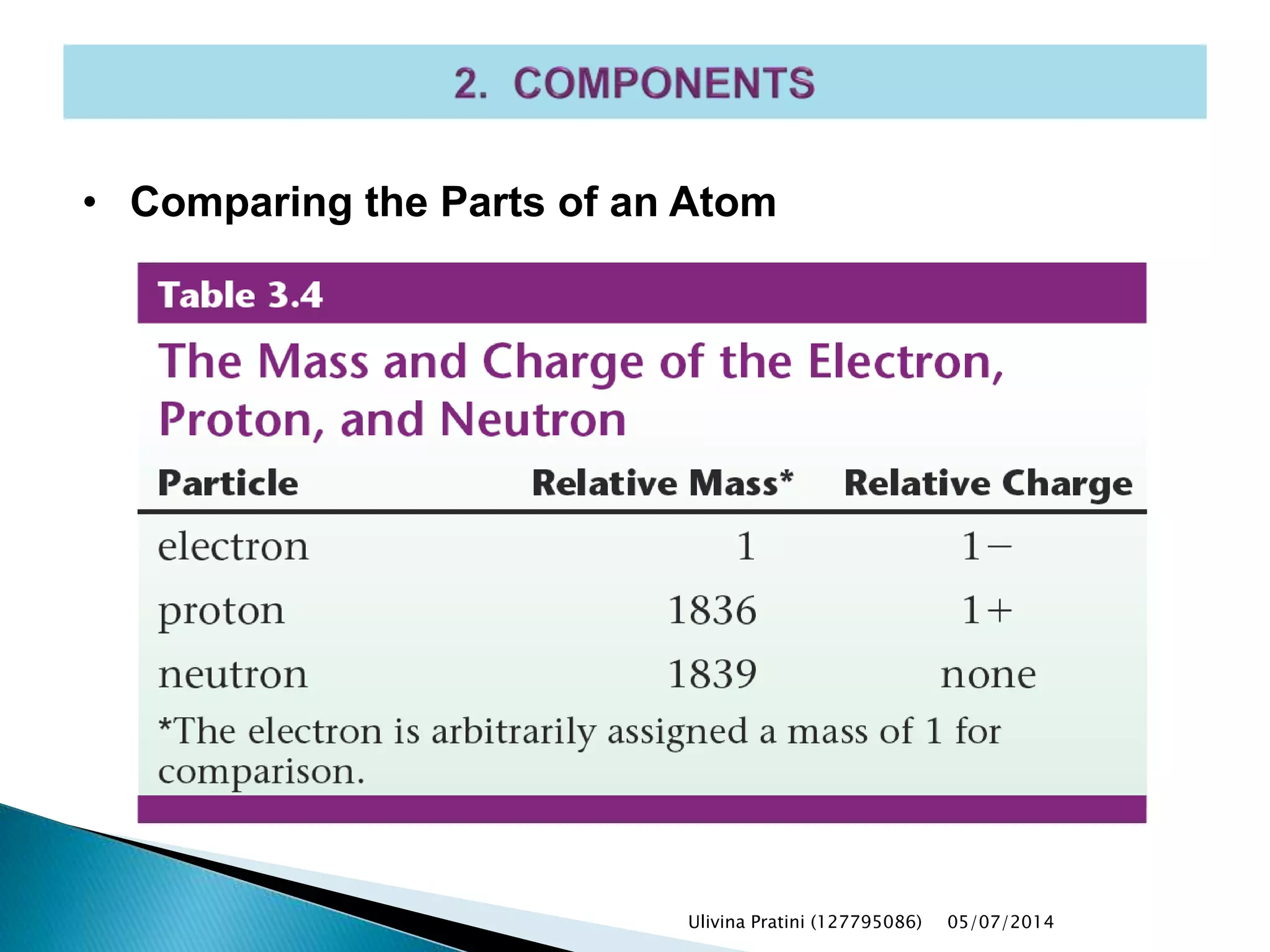
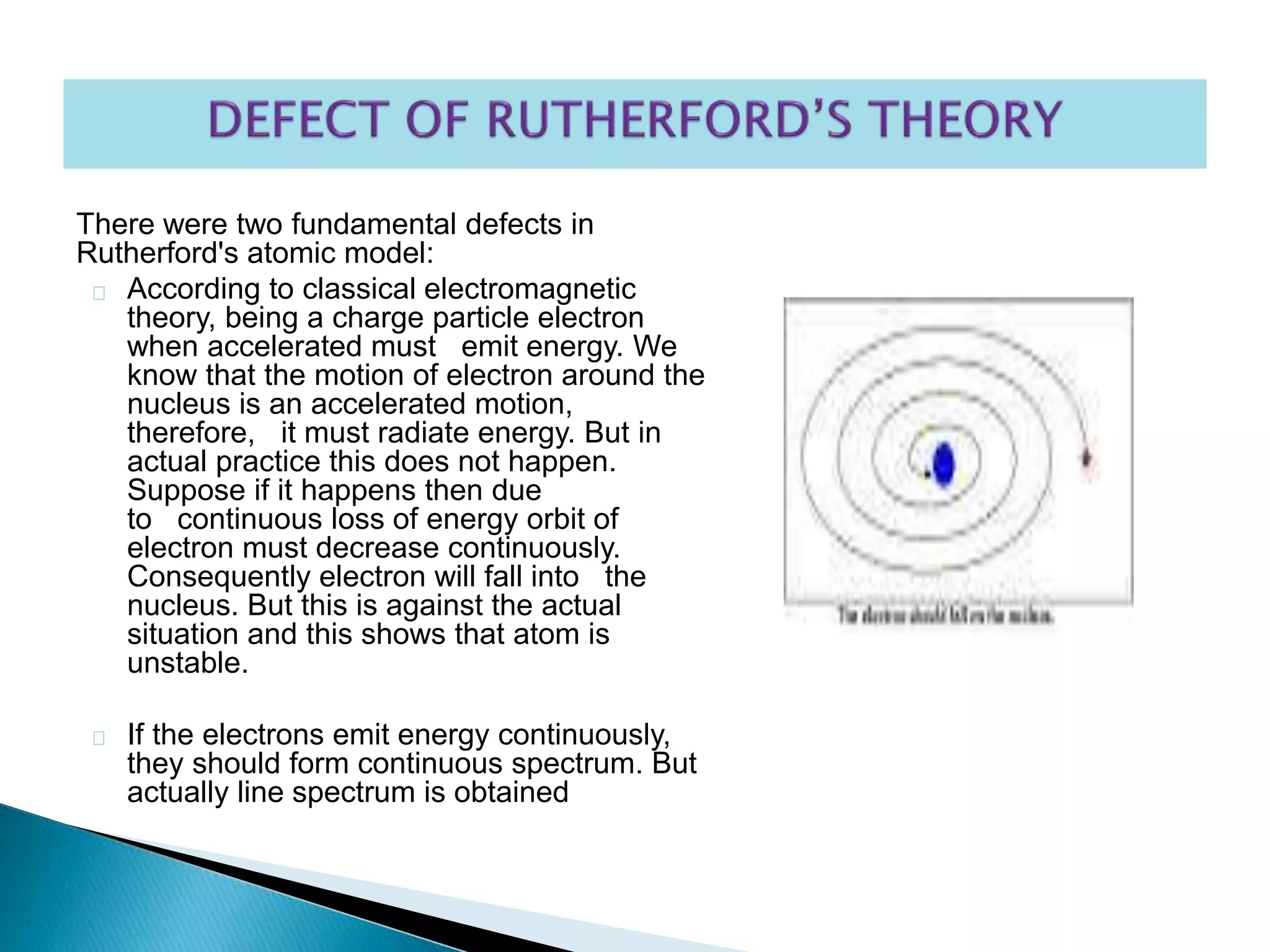
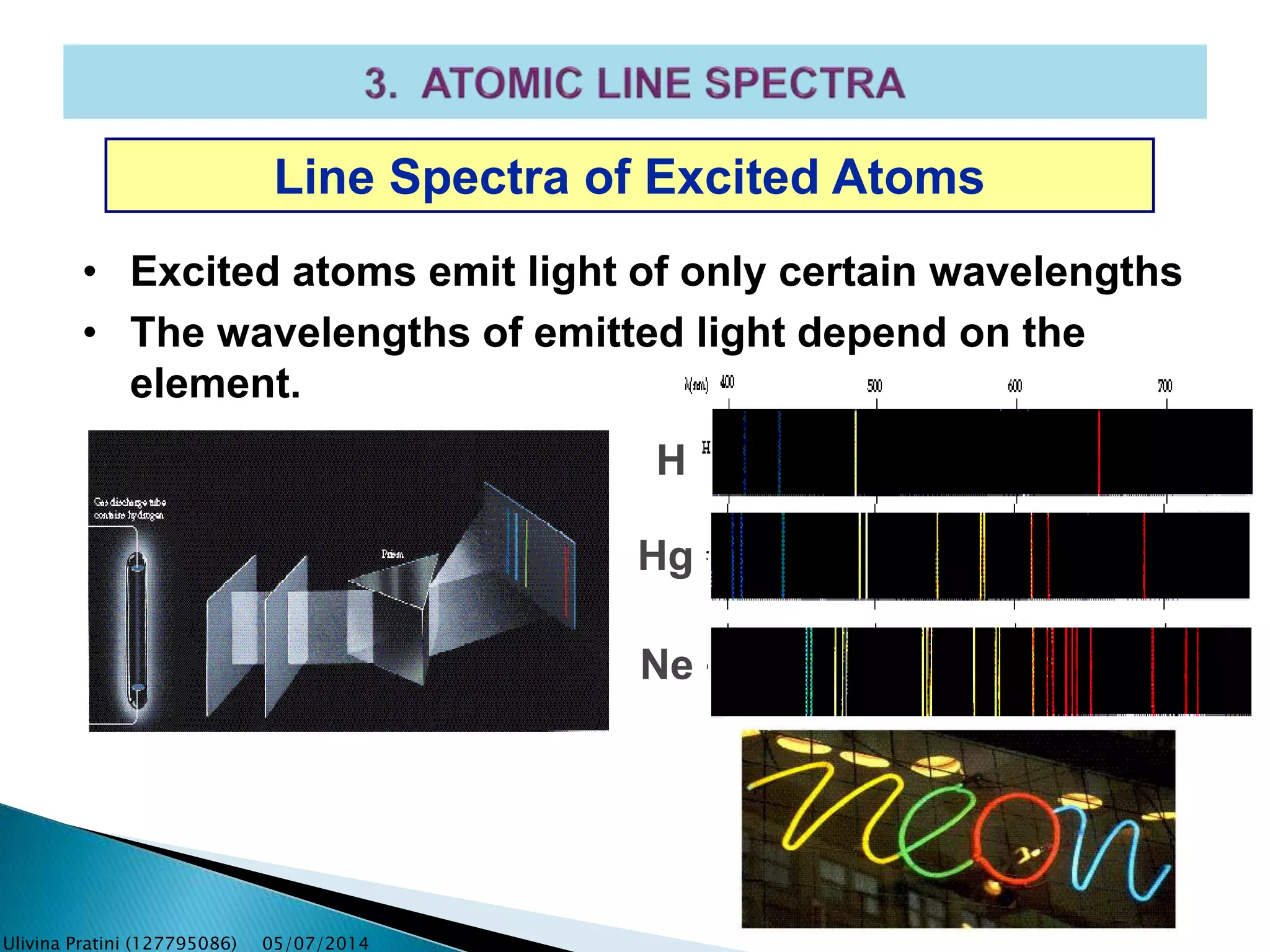
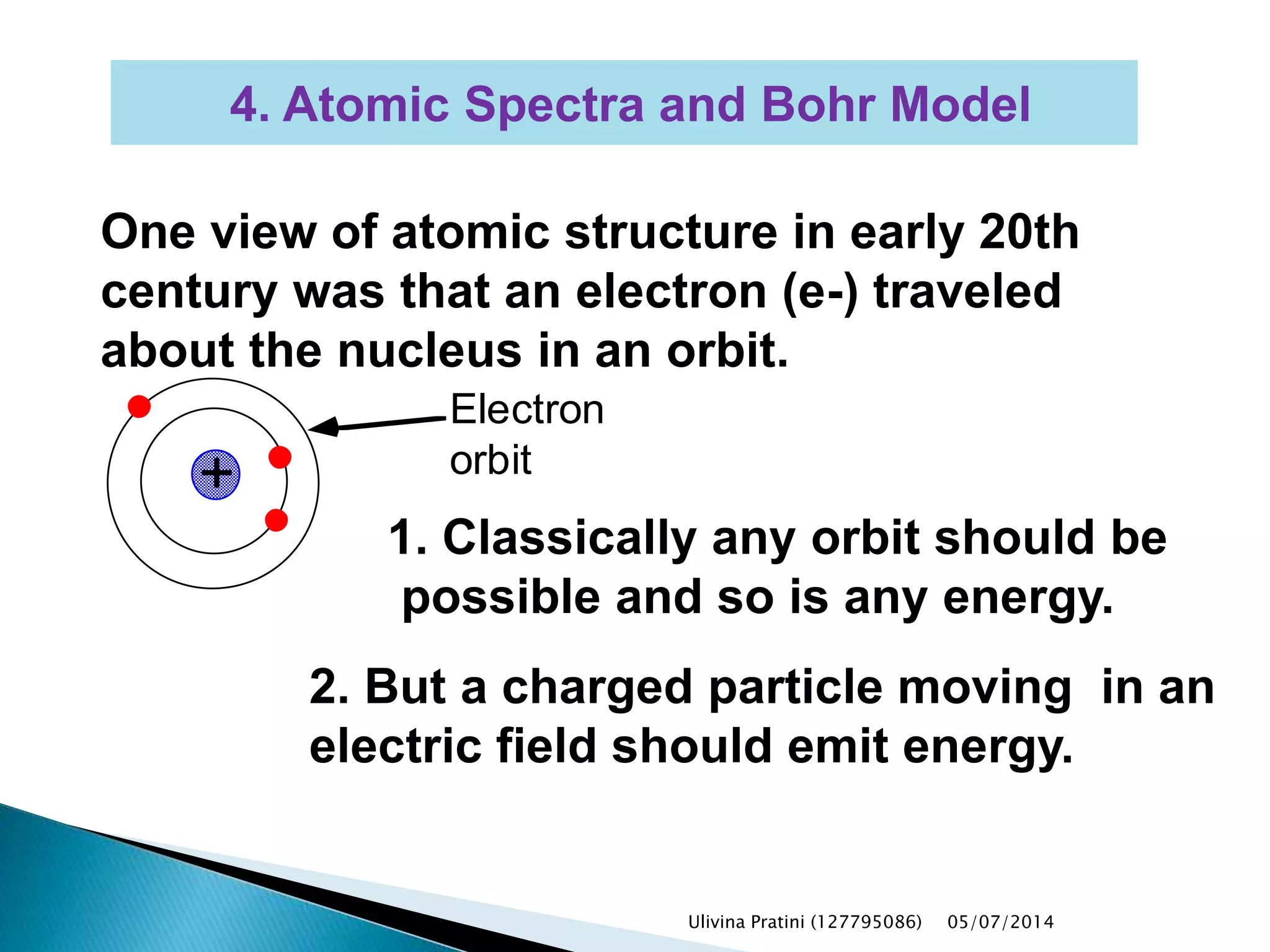
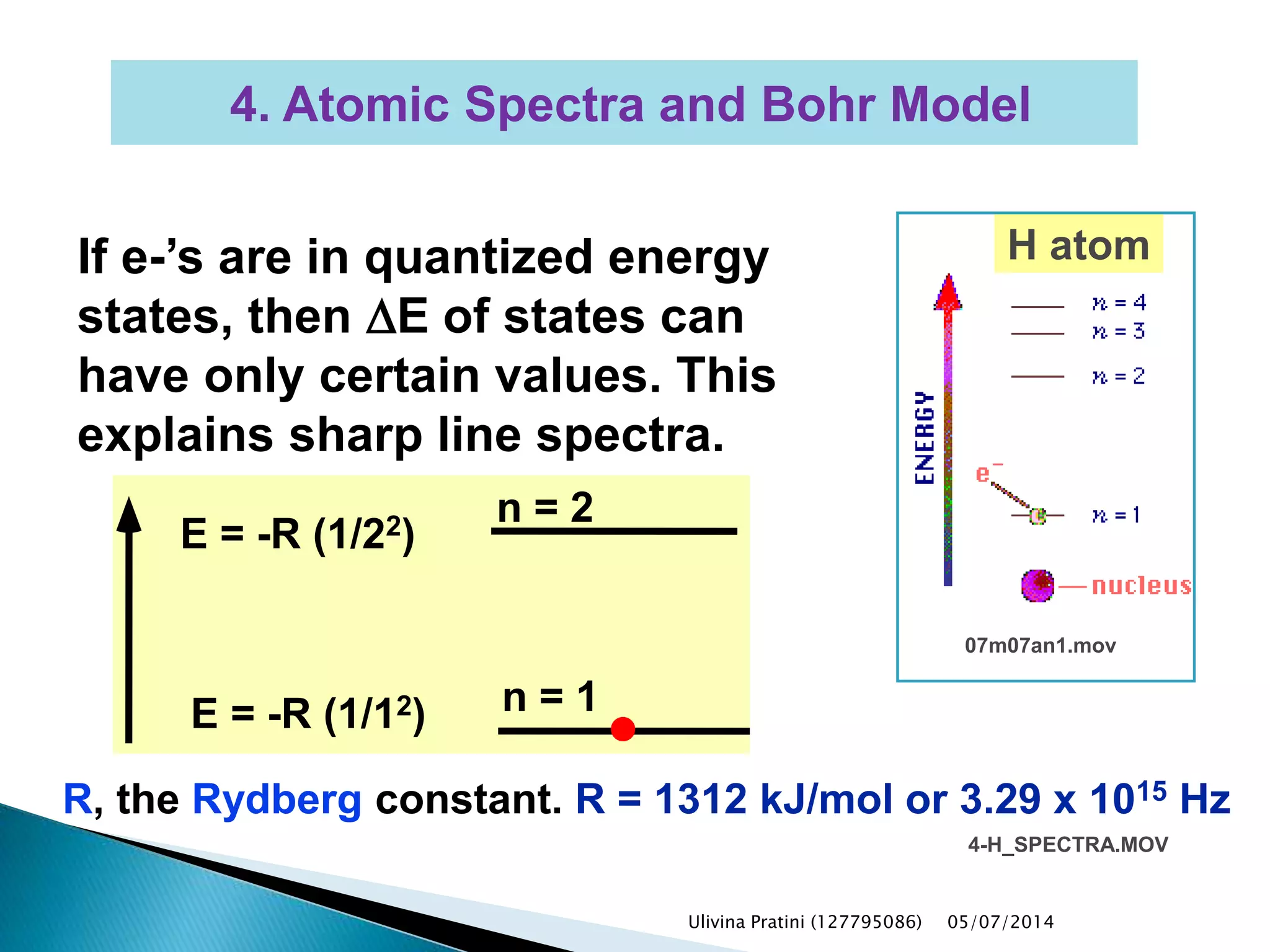
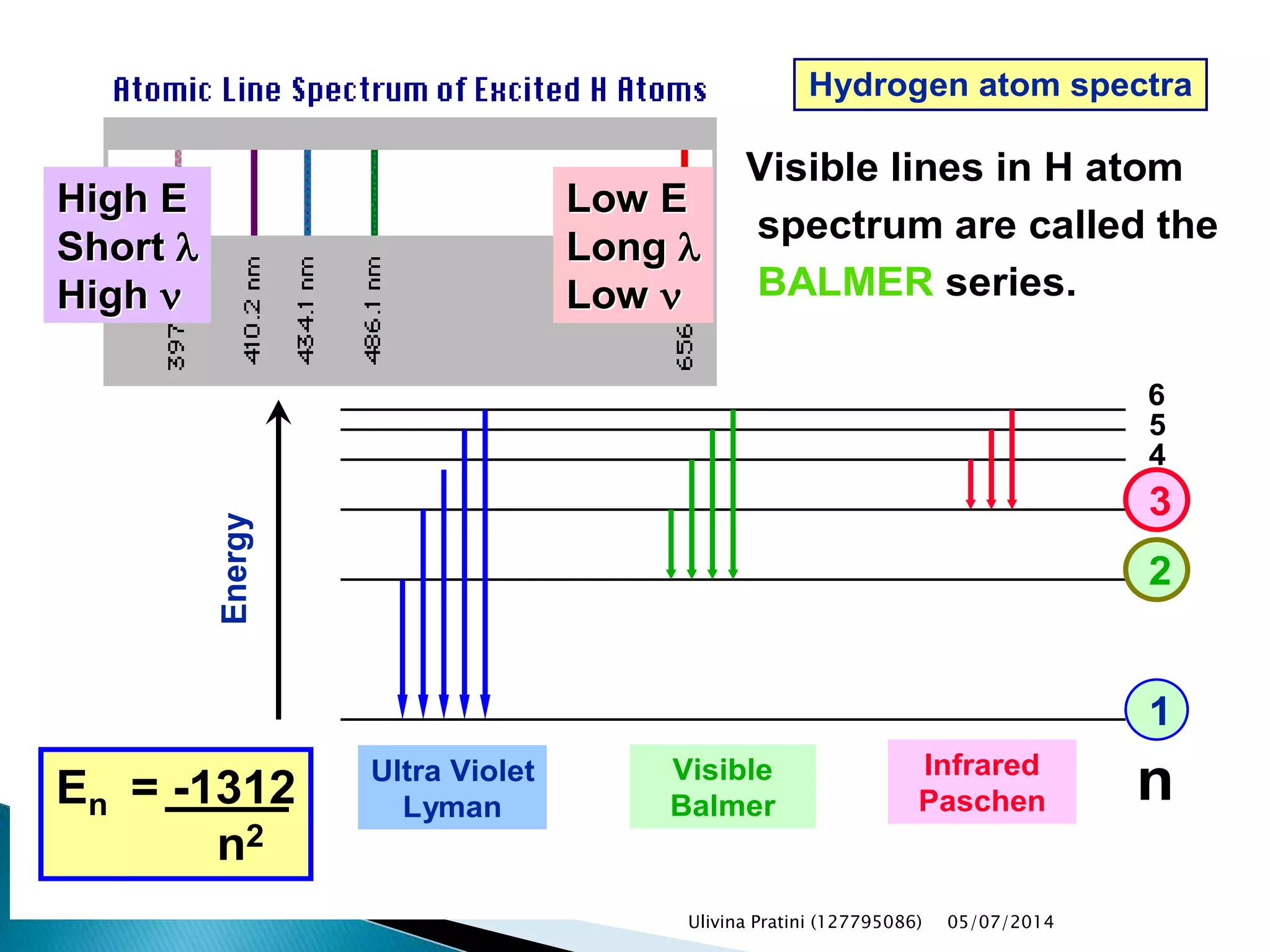
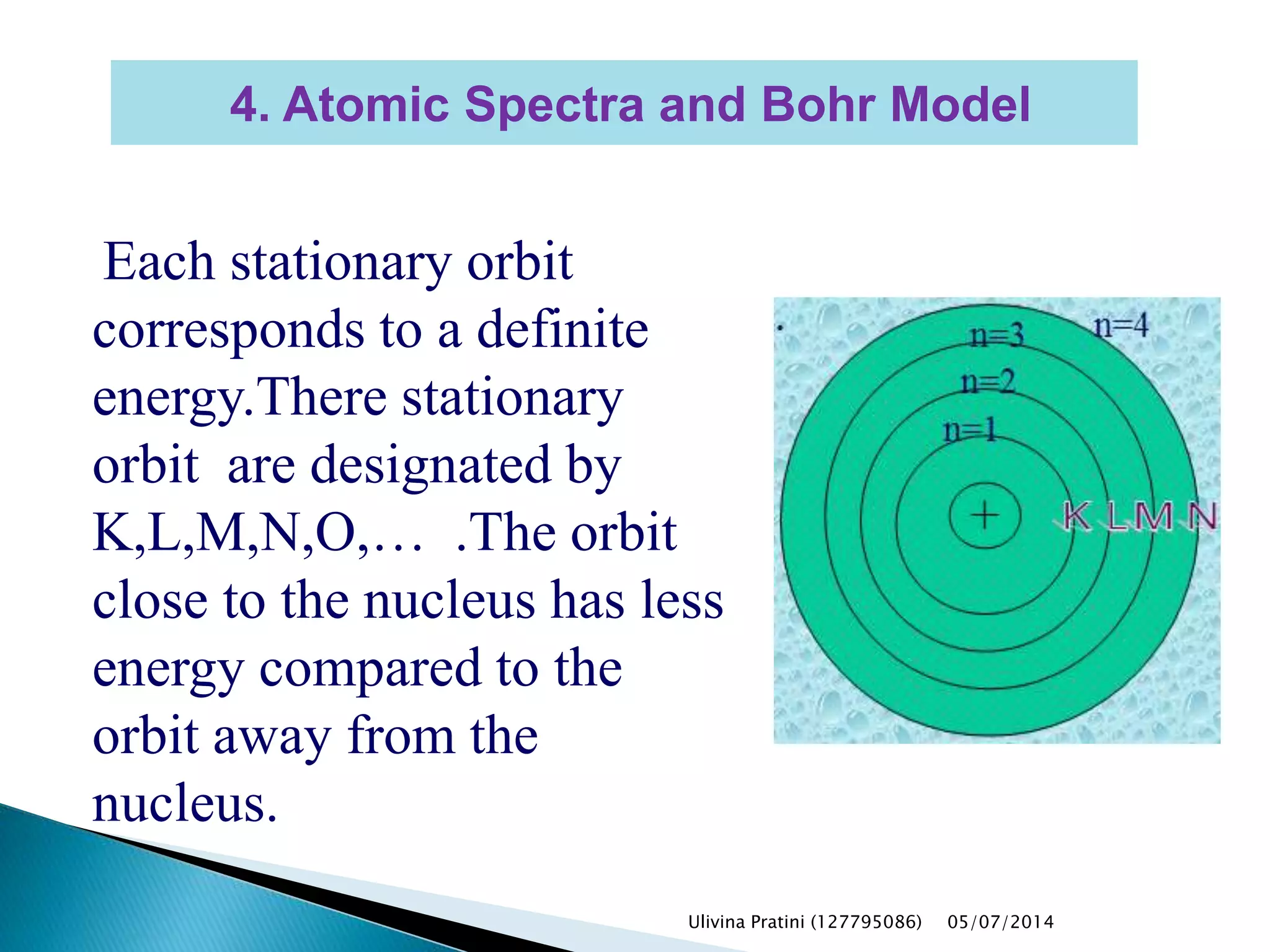
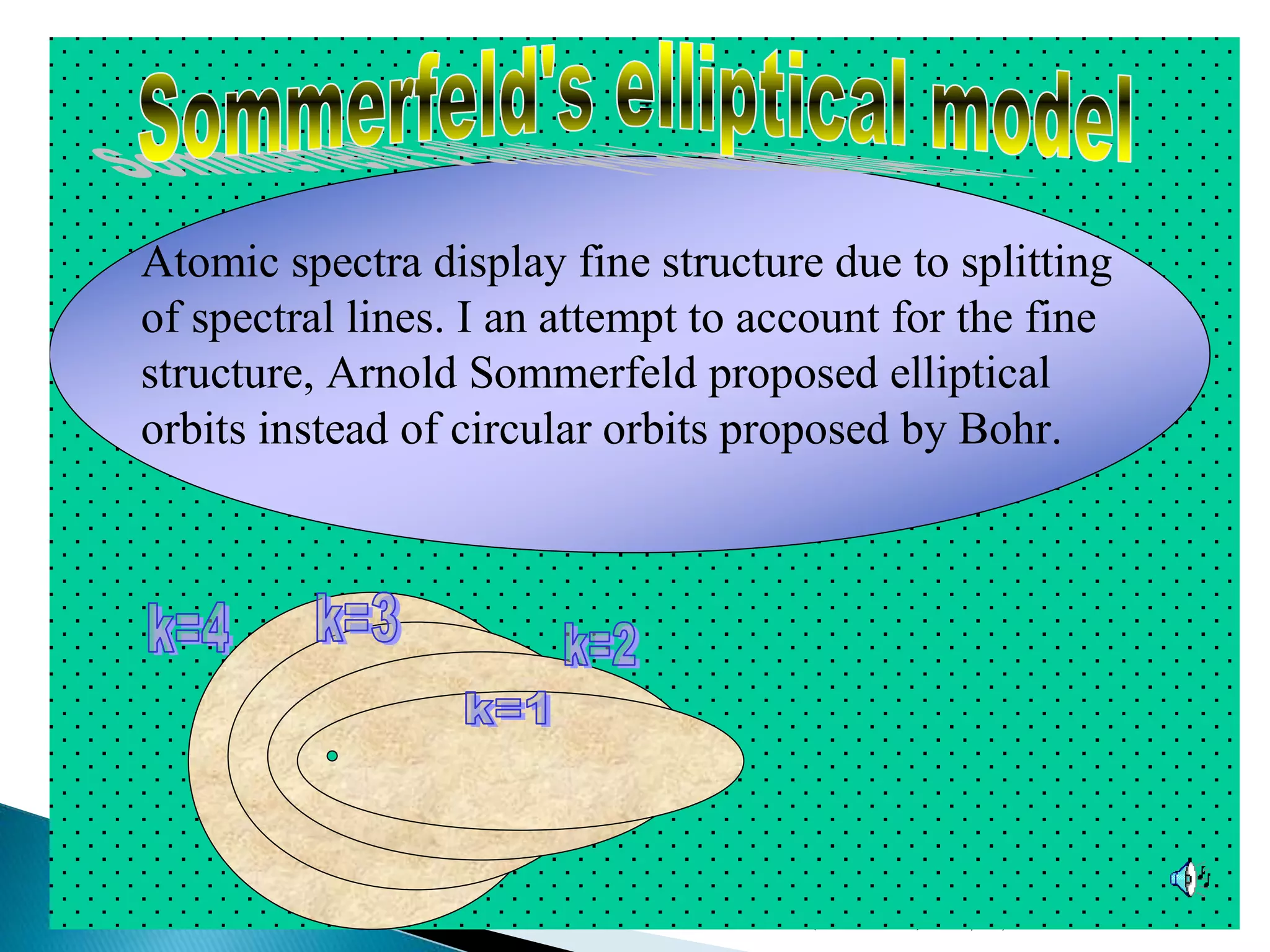
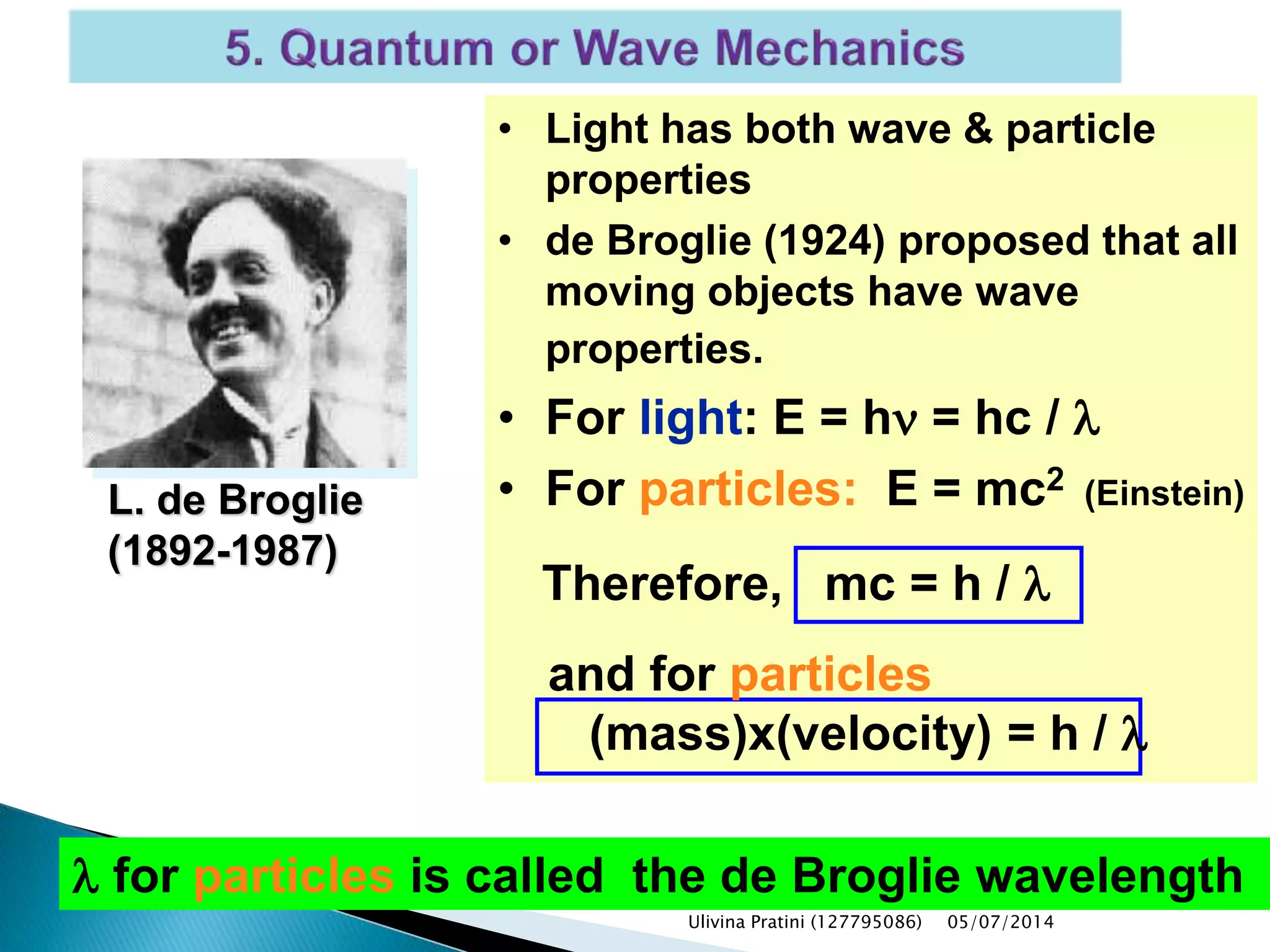
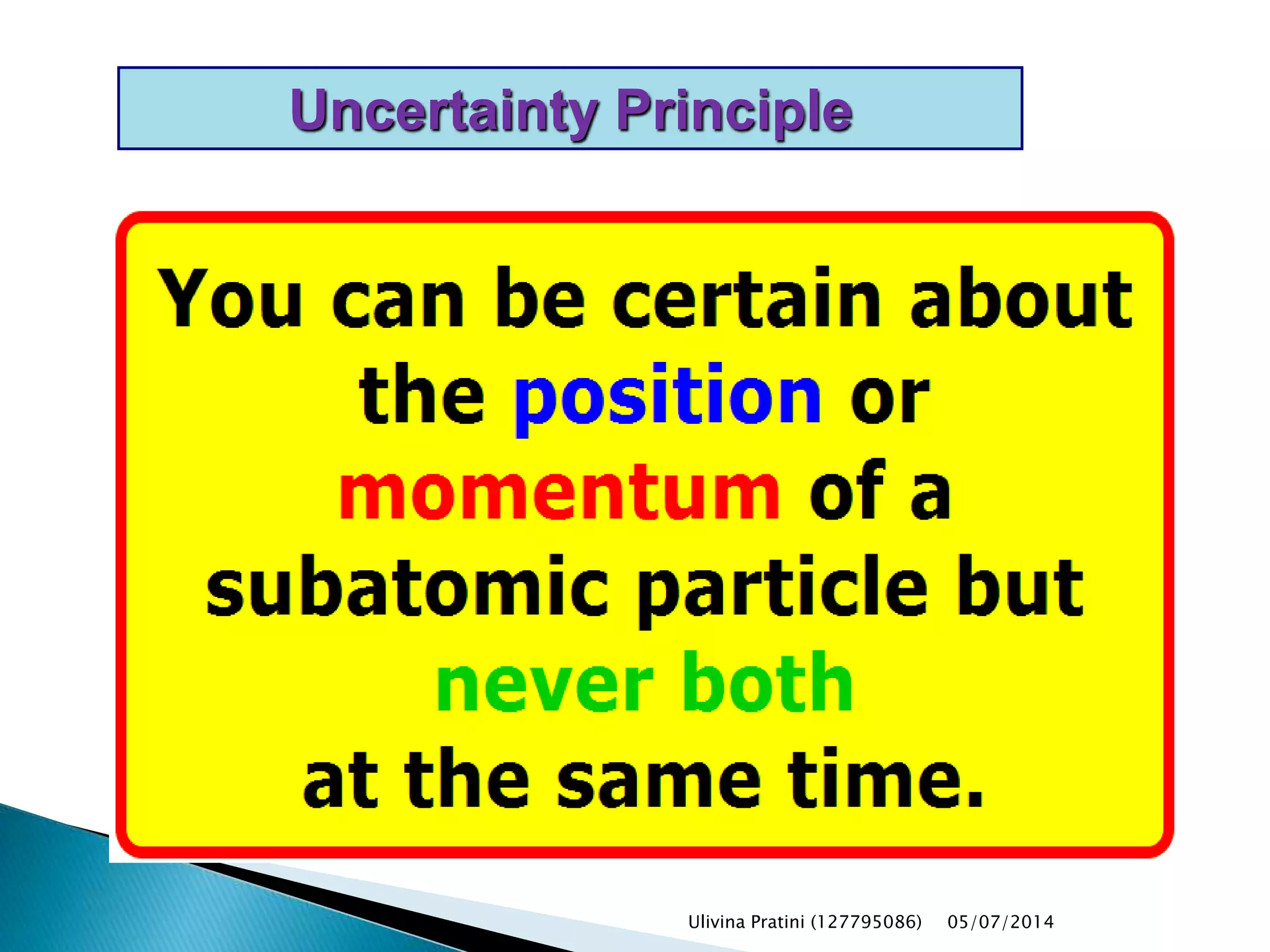
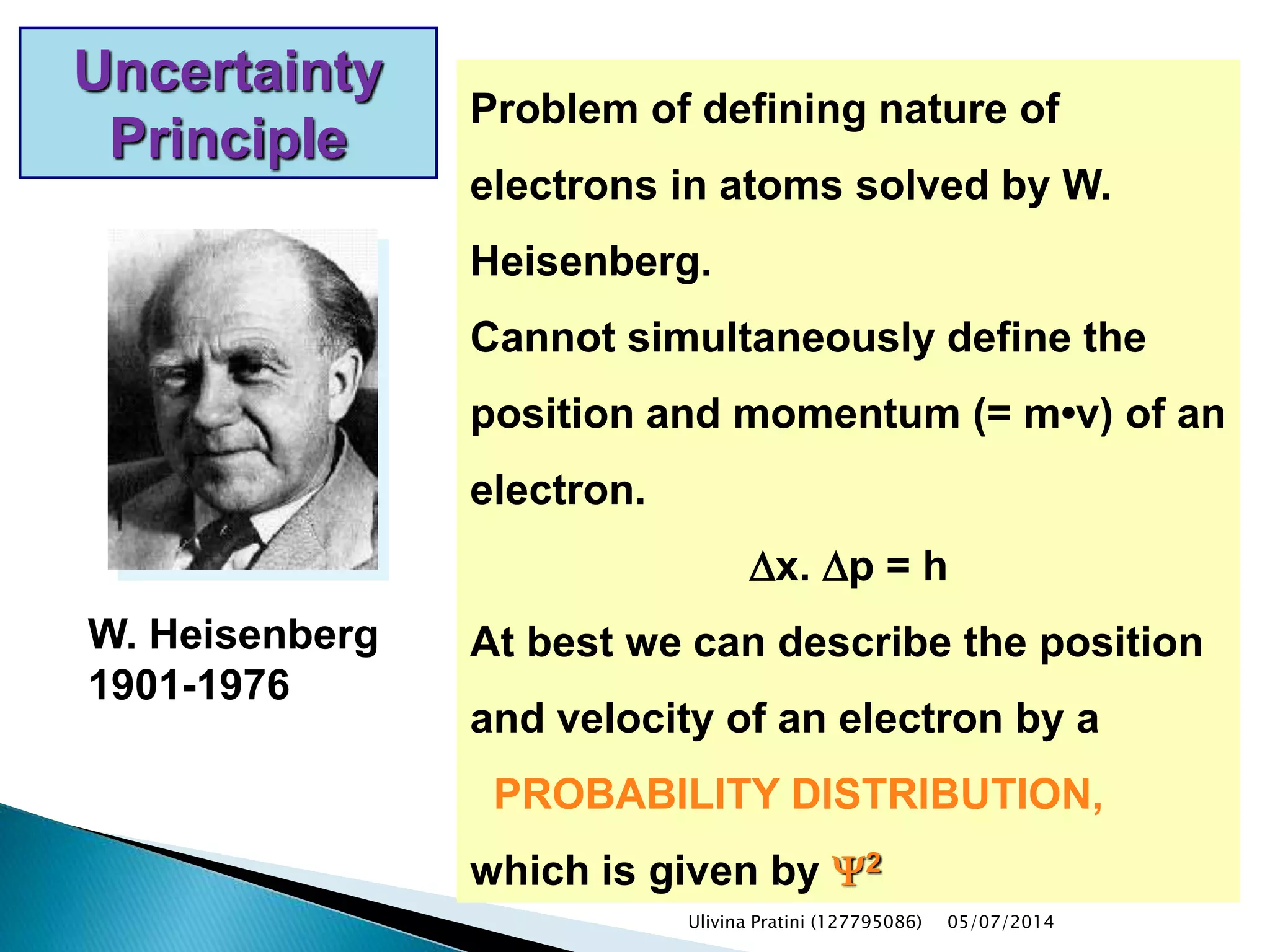
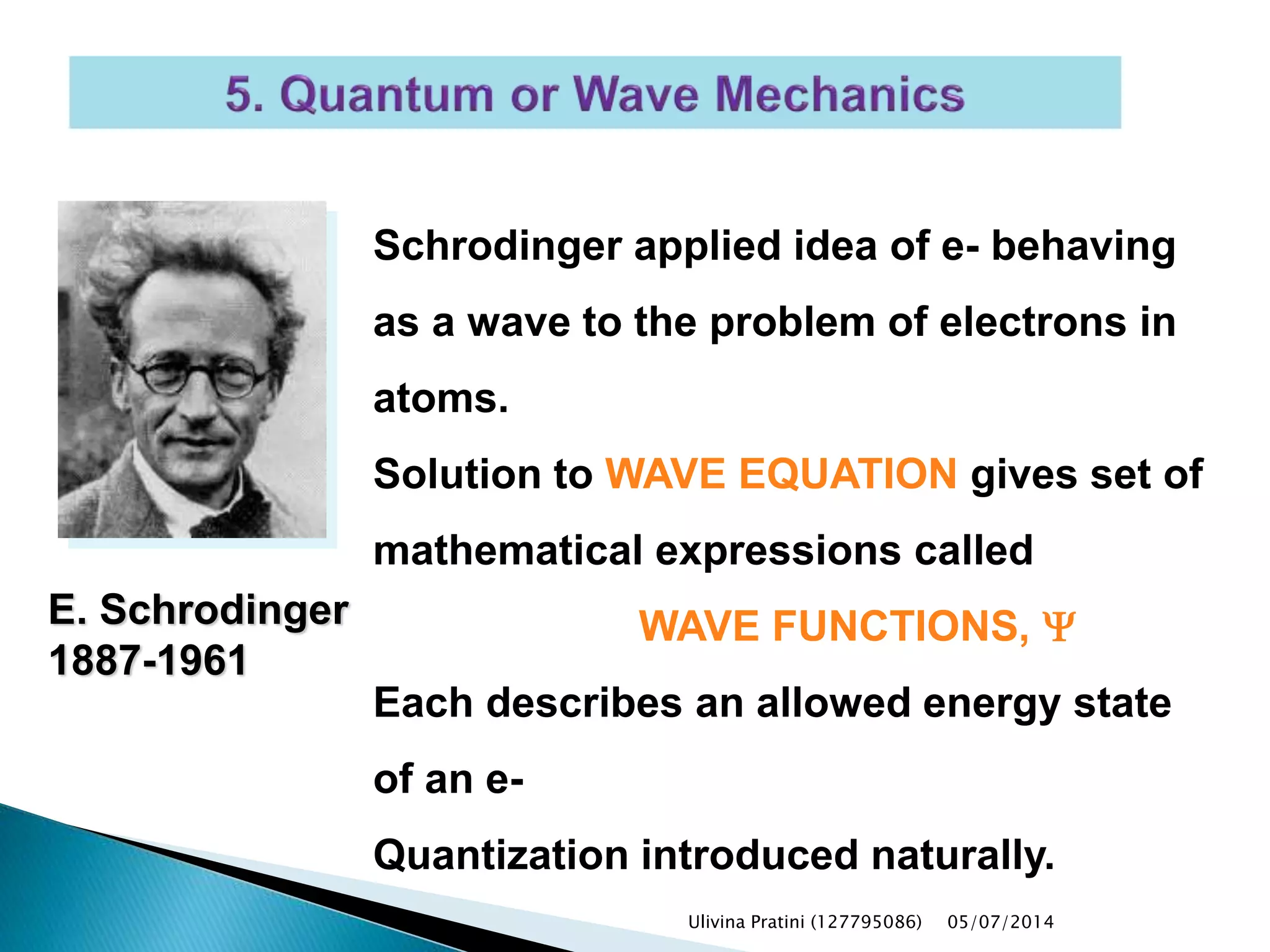
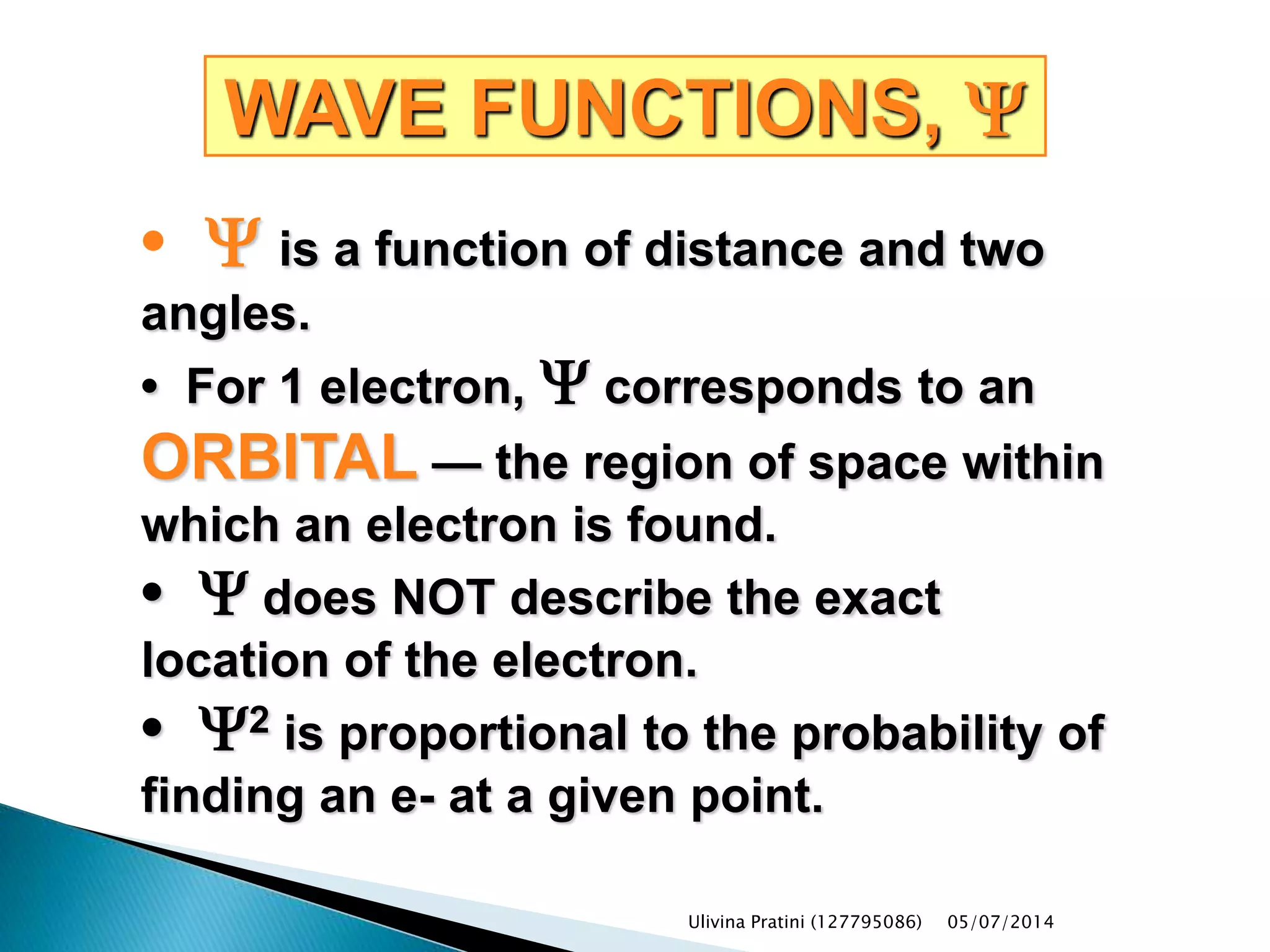
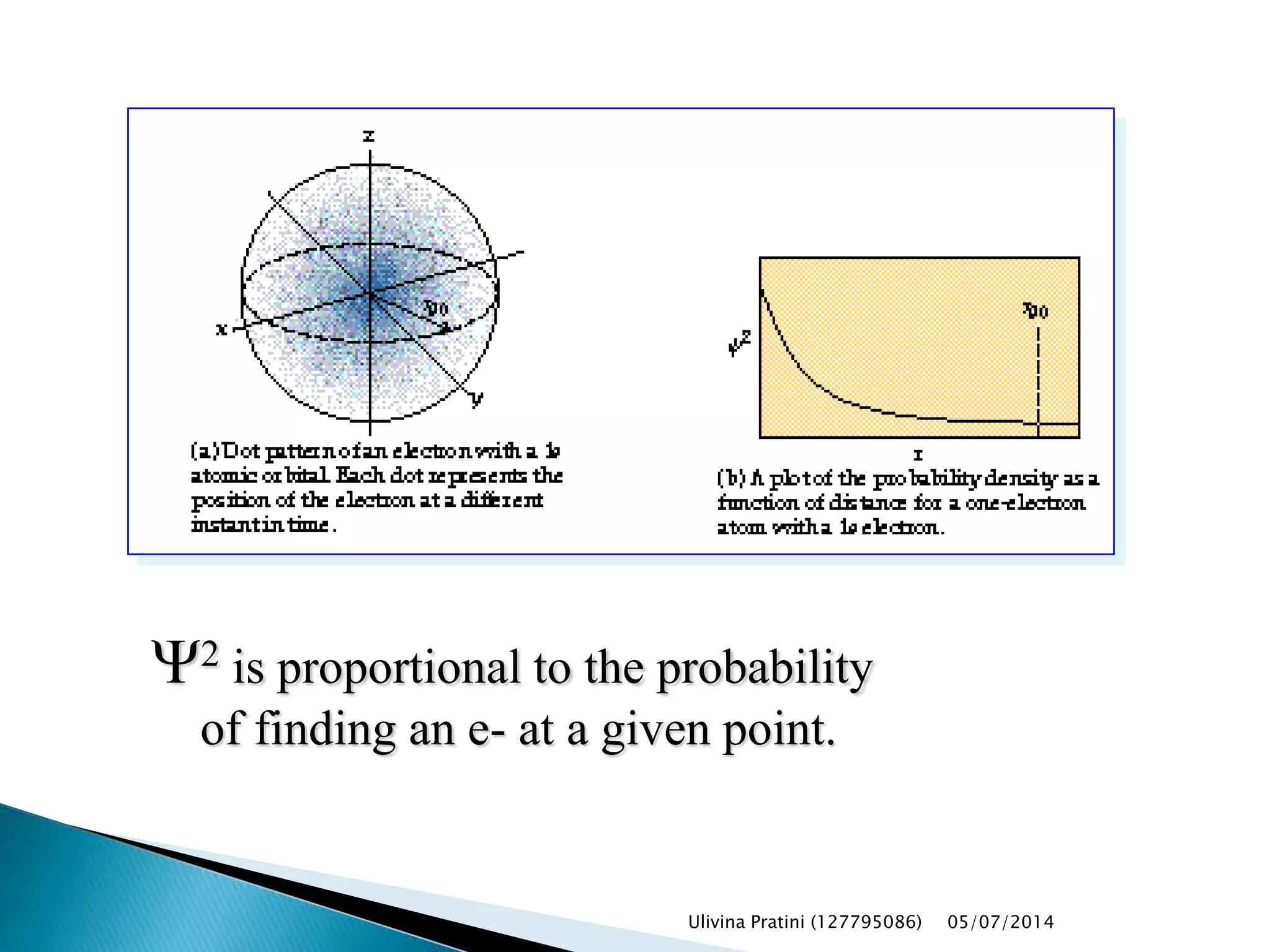
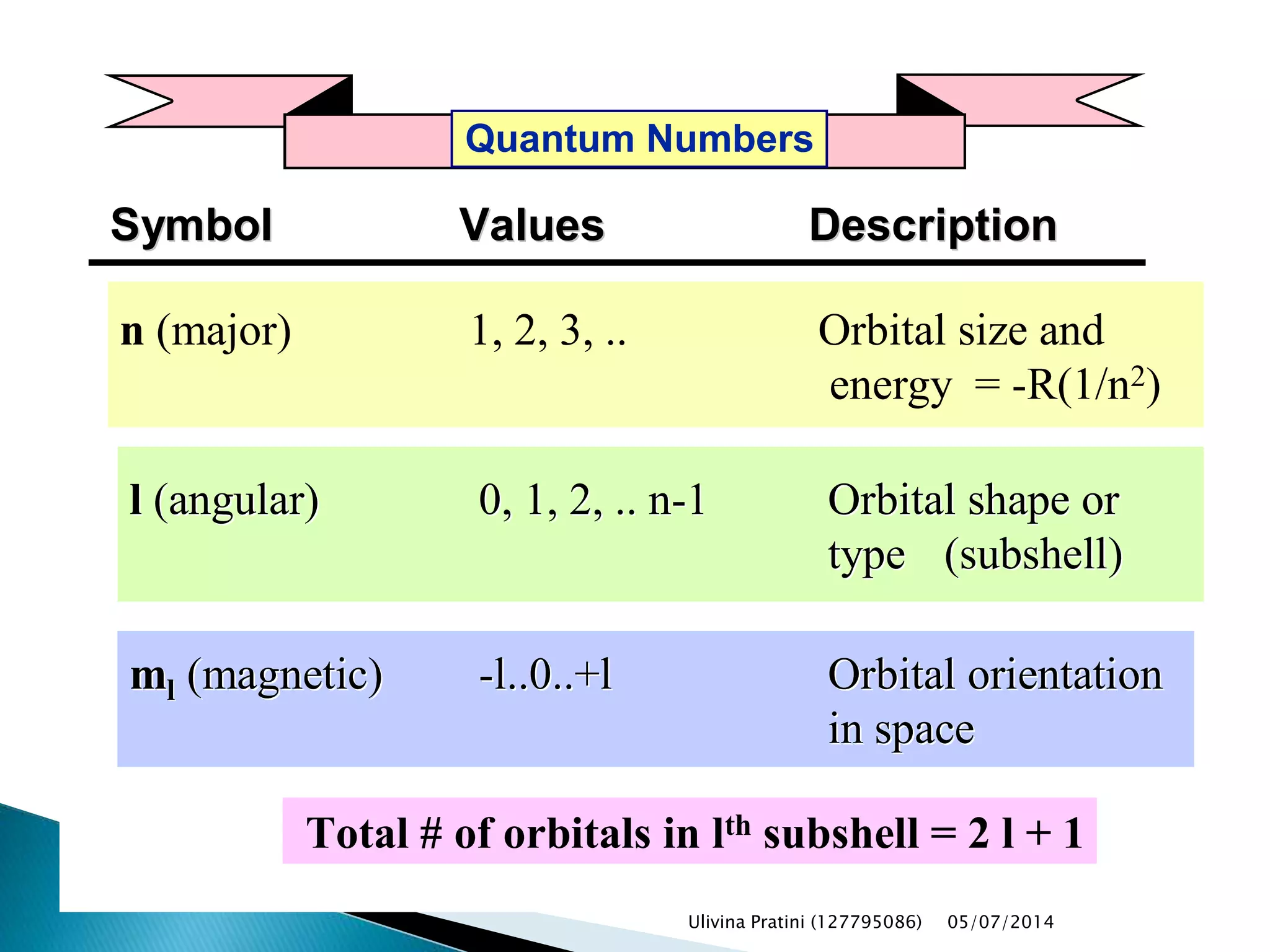
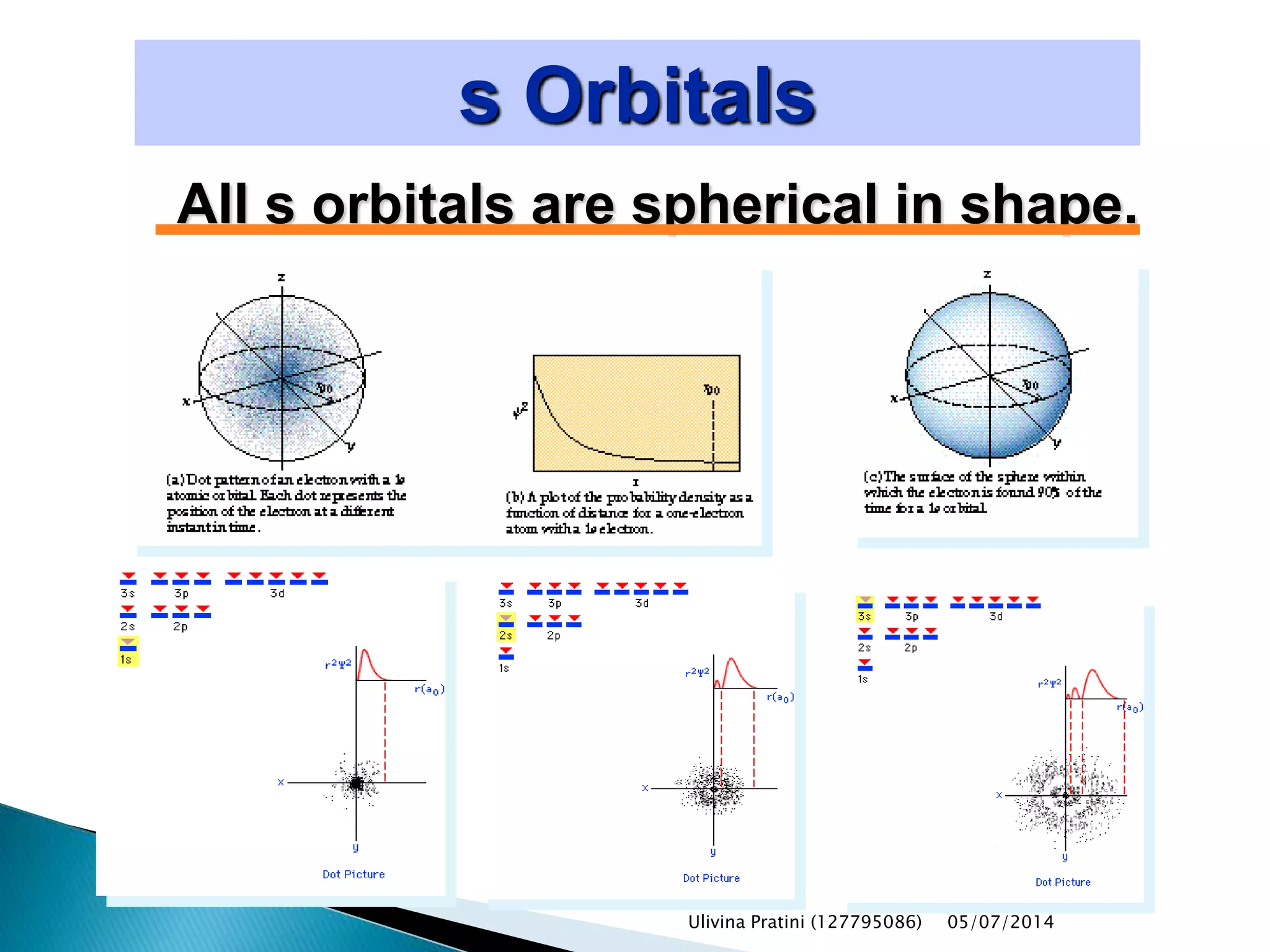
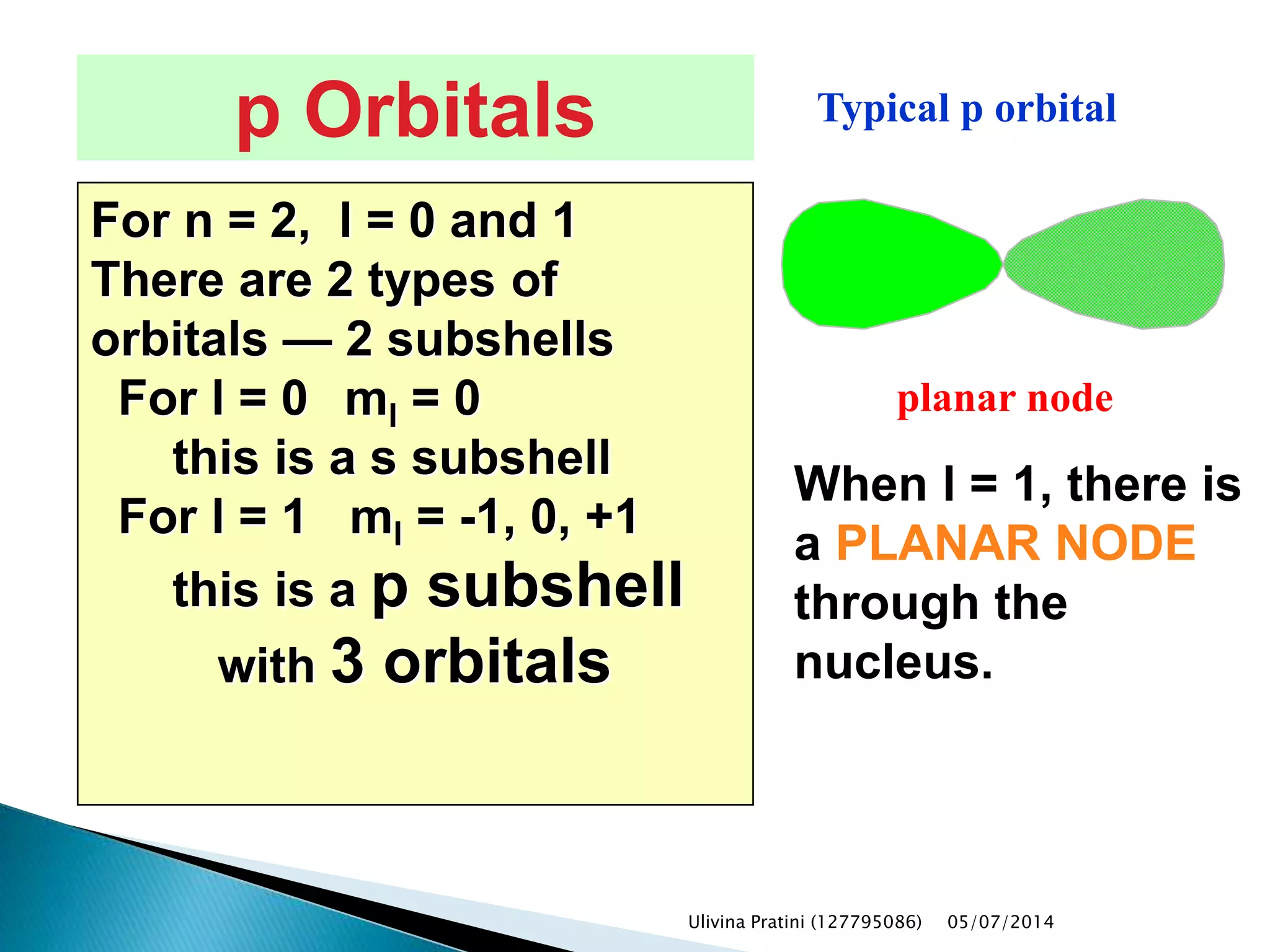
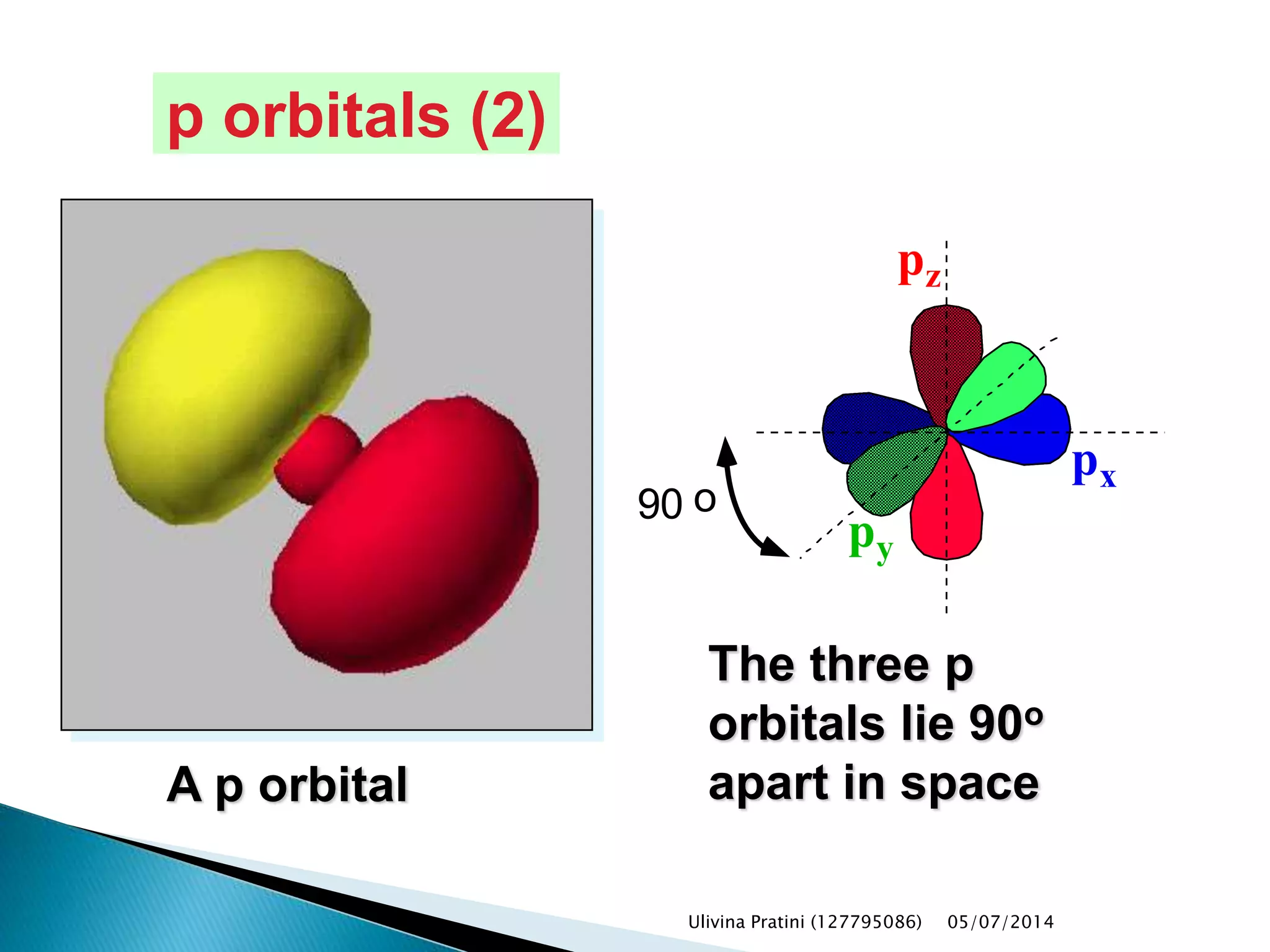
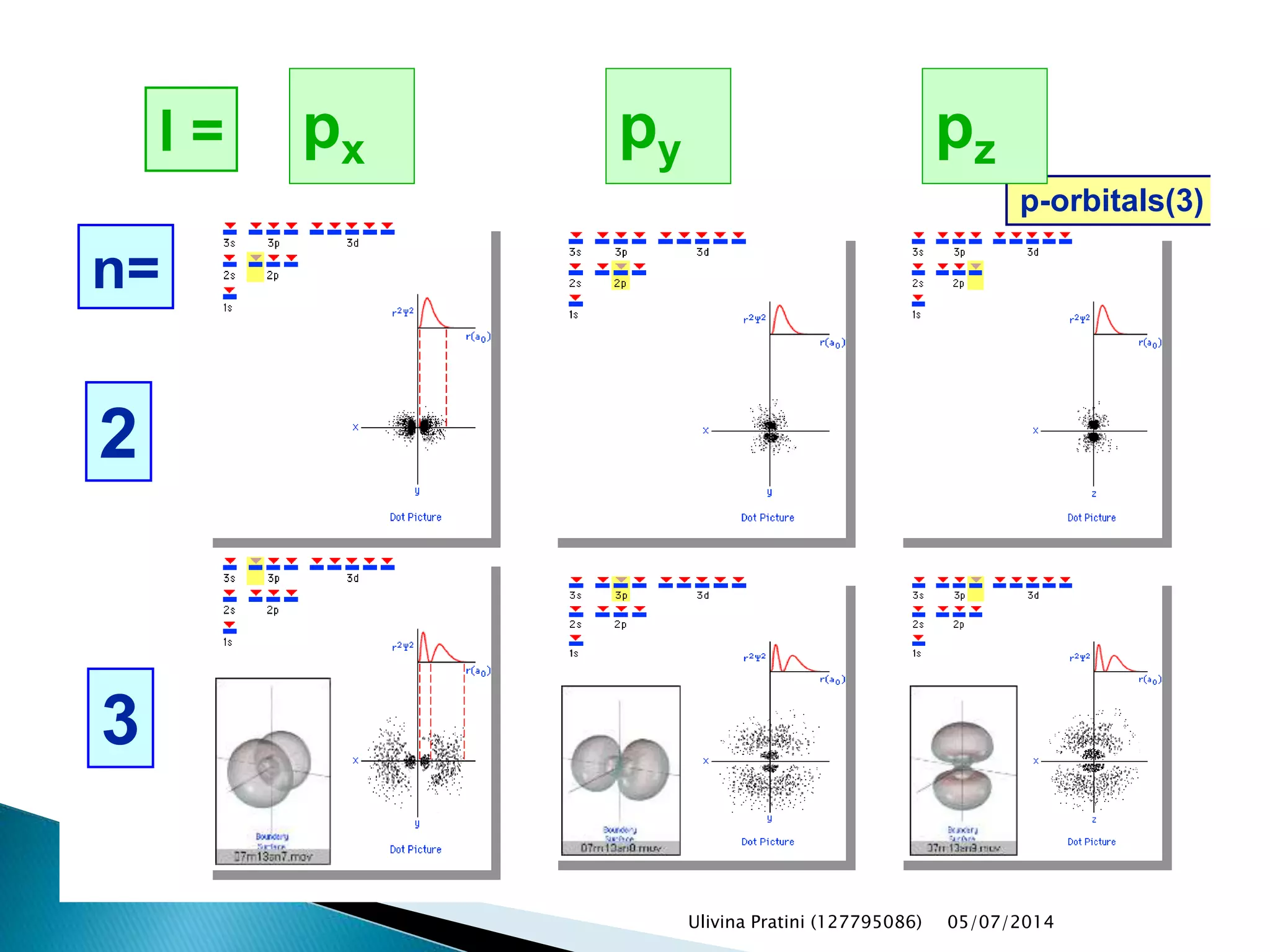
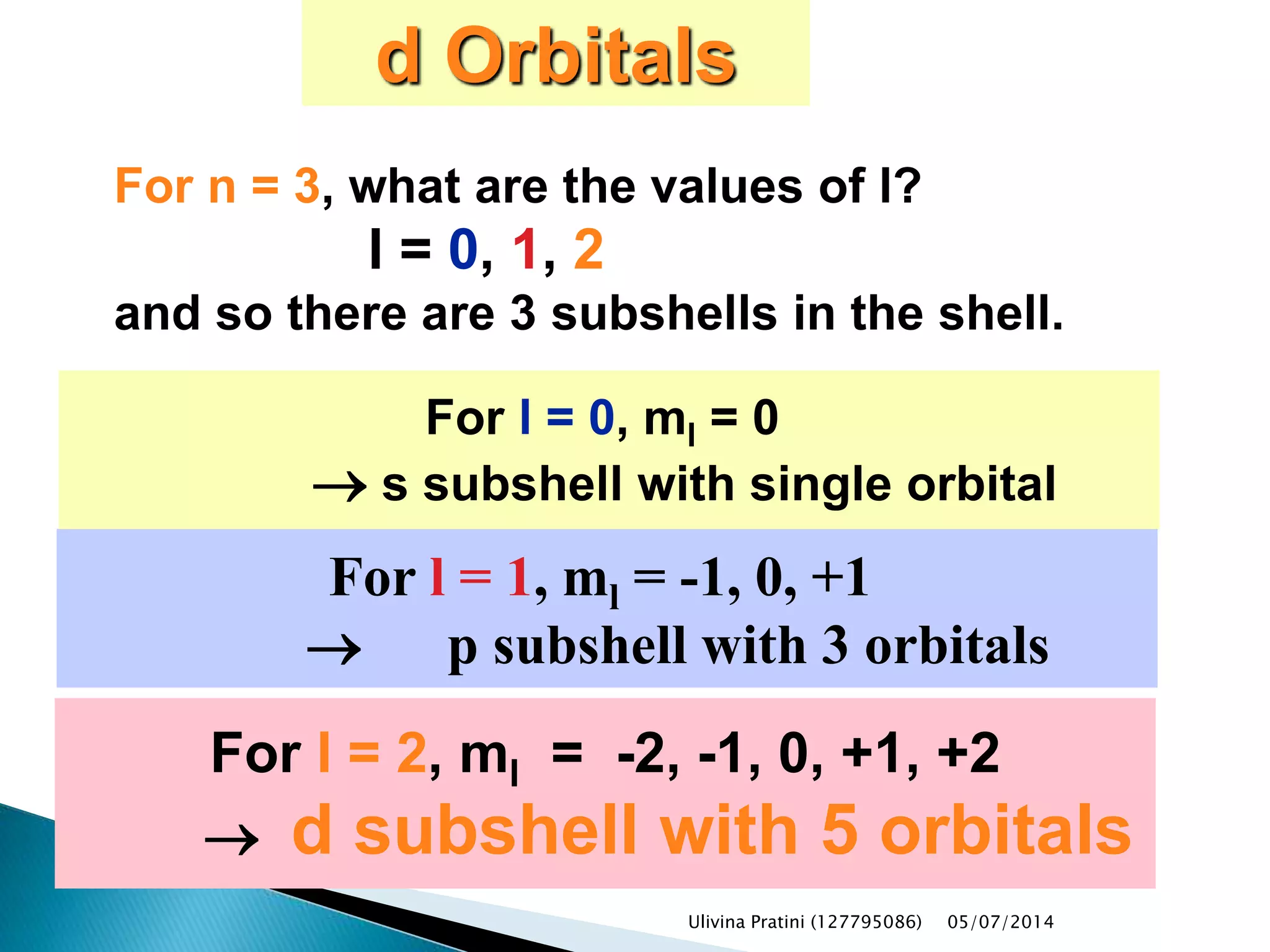
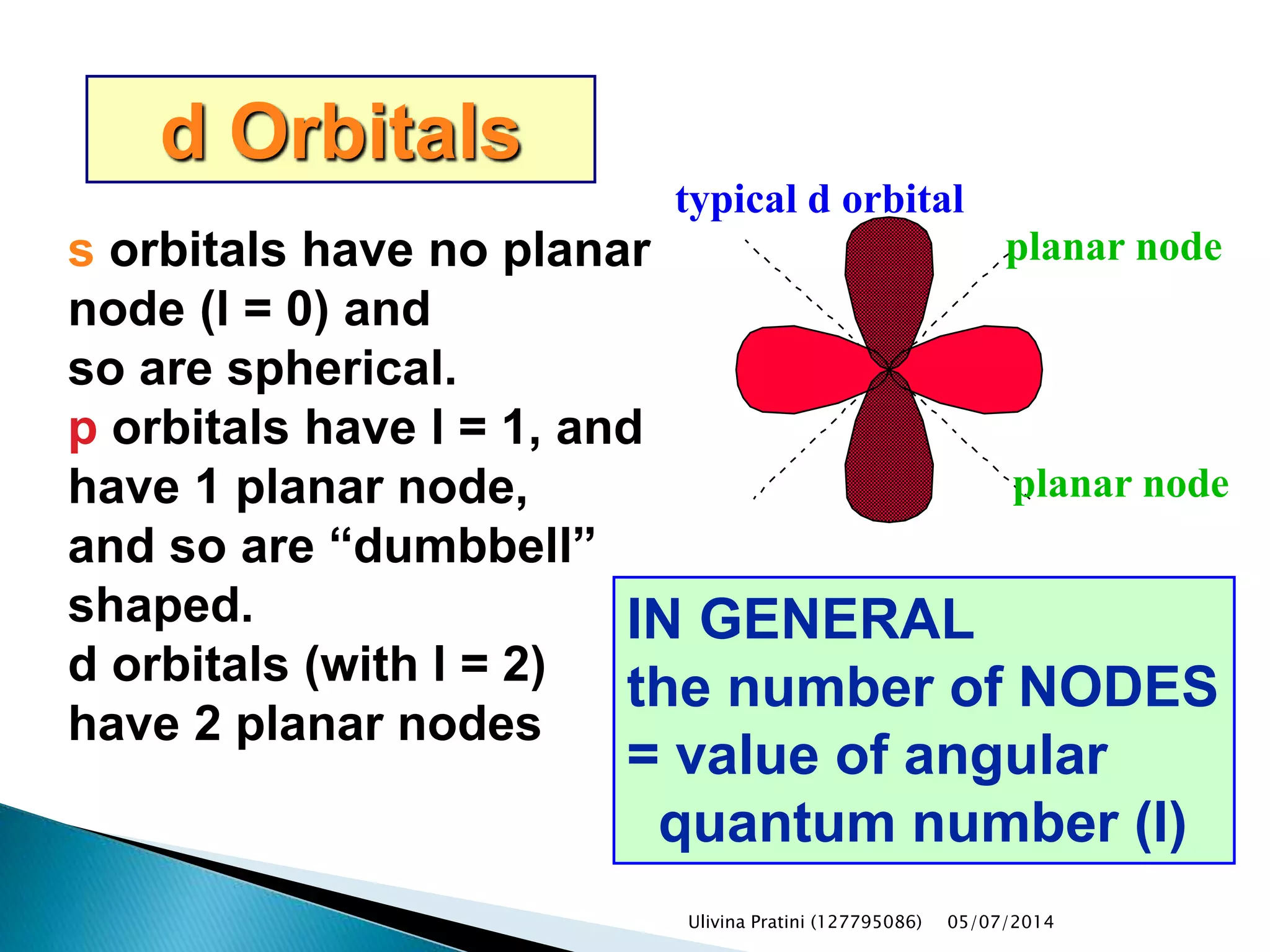
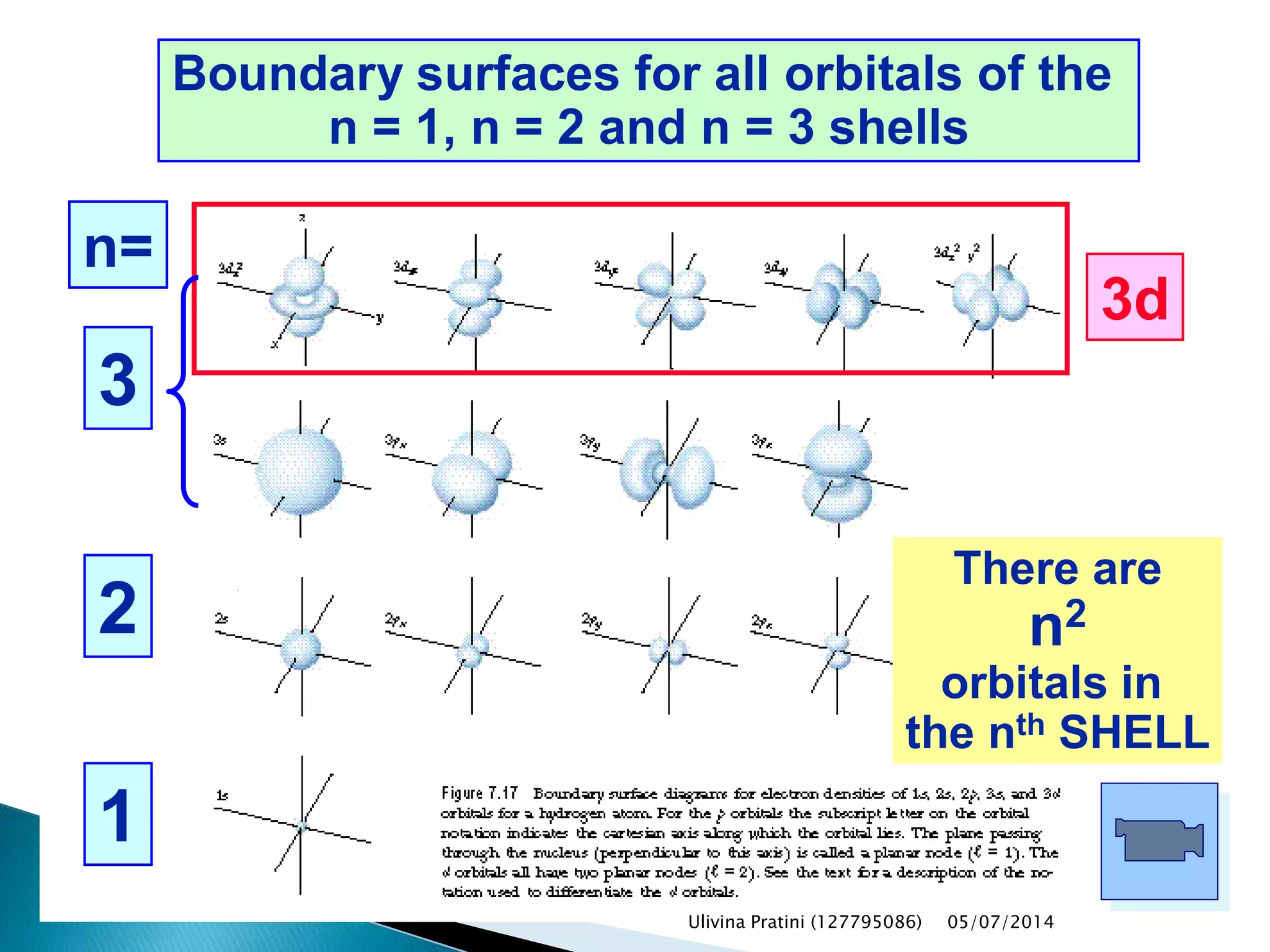
This document provides an overview of the history of atomic structure models. It begins with early Greek philosophers' concept of atoms. In the 19th century, Dalton proposed that elements are made of unique atom types that can combine. Experiments by Thomson, Rutherford and Bohr led to discoveries about the electron and nuclear structure of atoms. Bohr's 1913 model improved on Rutherford's by proposing discrete electron orbits. Later, the developments of quantum mechanics by Heisenberg, Schrodinger, de Broglie and others explained atomic structure and spectra through wave functions and quantum numbers. This allowed visualization of electron orbitals in atoms.