Embed presentation
Downloaded 62 times
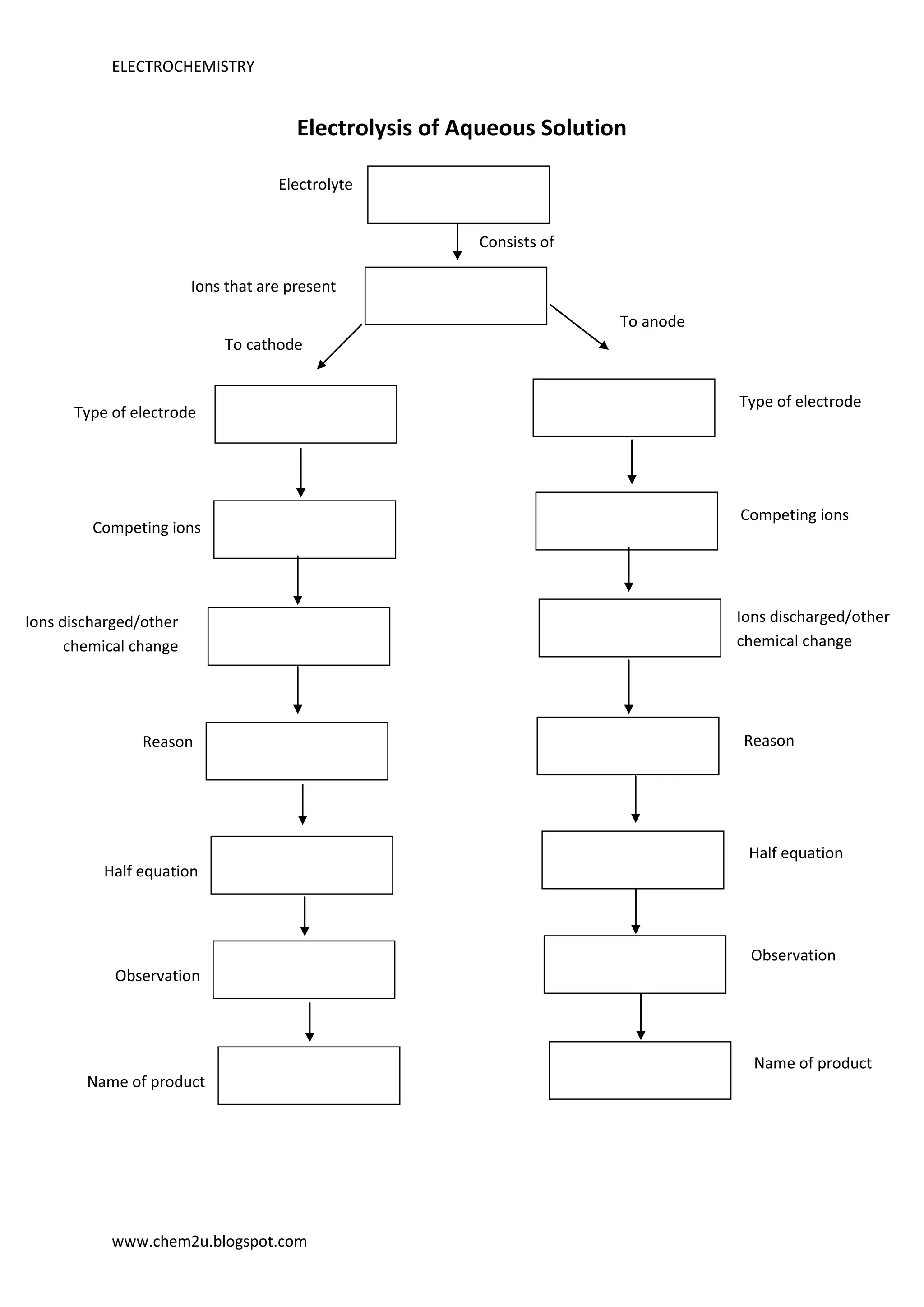
Electrolysis is the process of using electric current to drive nonspontaneous redox reactions. During electrolysis of an aqueous solution, ions in the electrolyte migrate to the anode or cathode depending on their charge. The type of electrode and competing ions determine which ions are discharged or undergo other chemical changes at each electrode along with the products observed.
