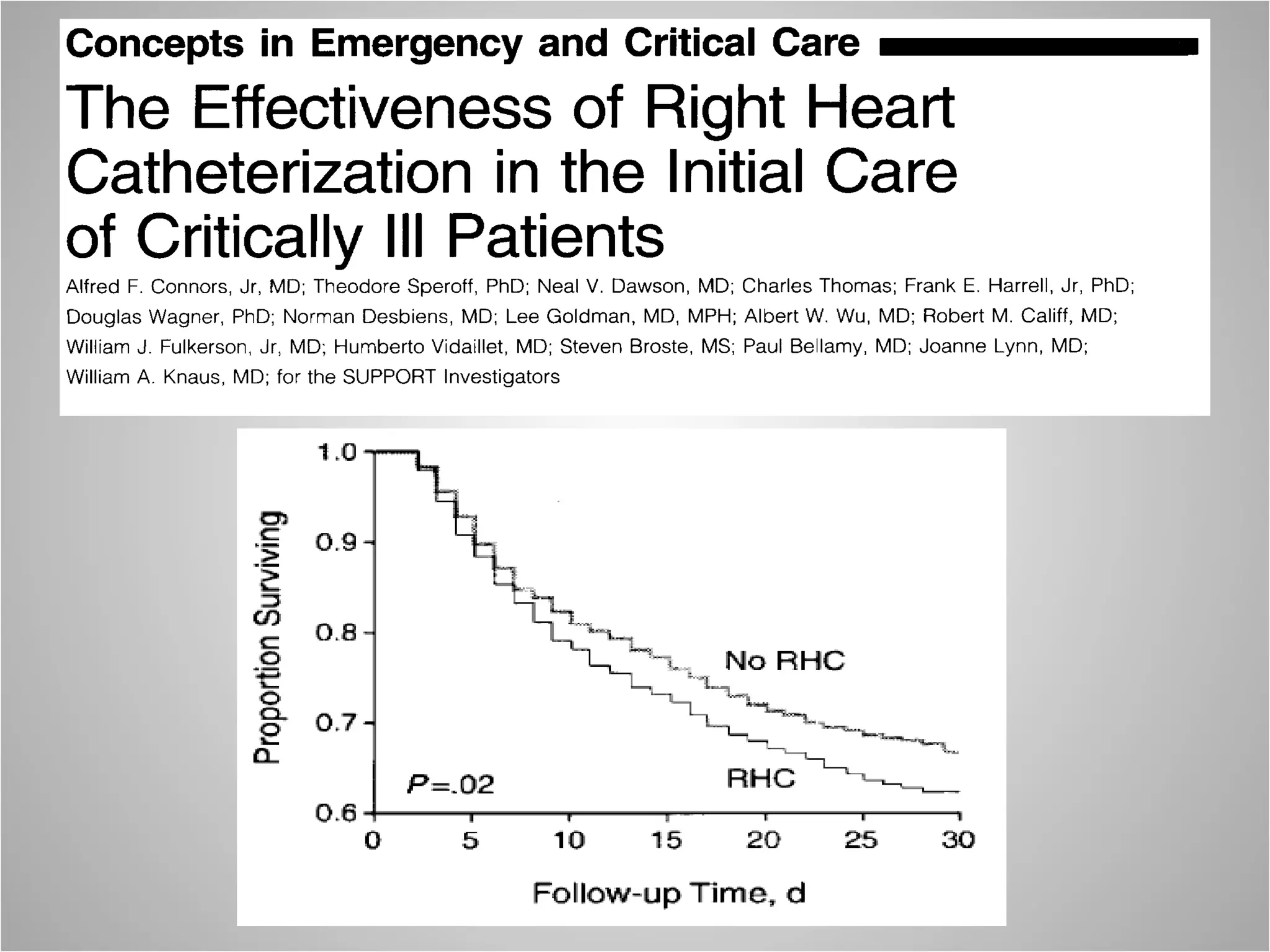
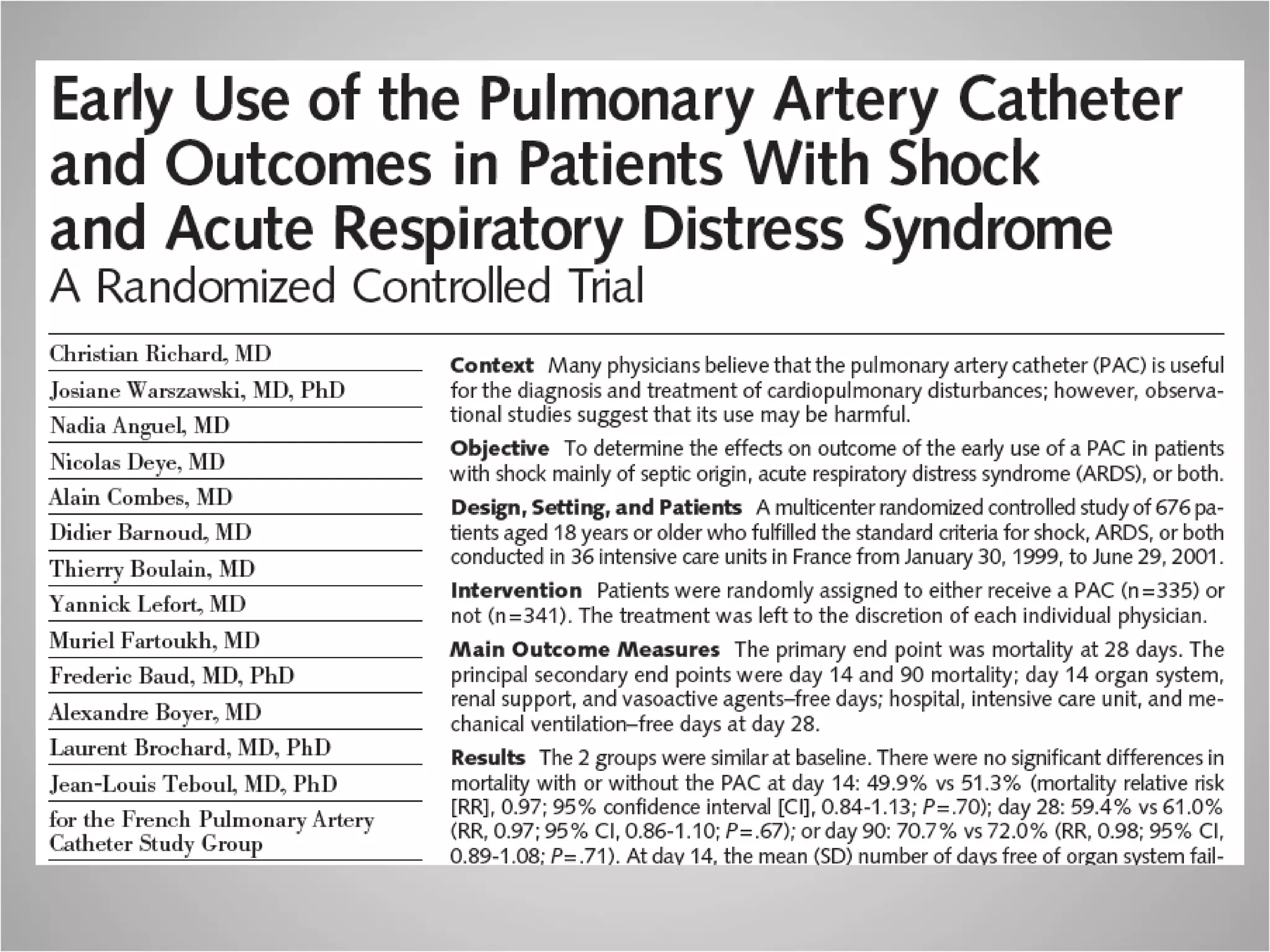
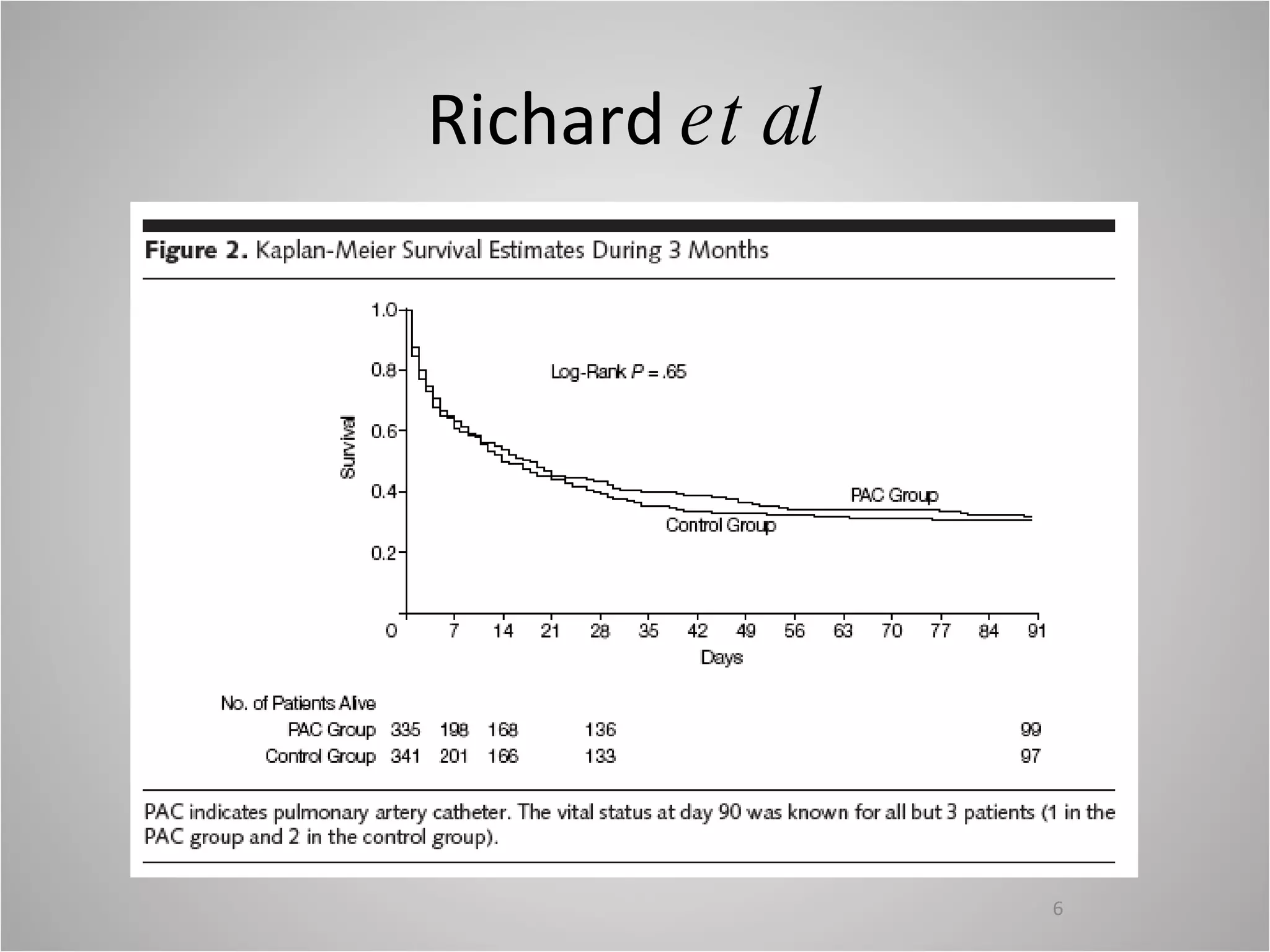
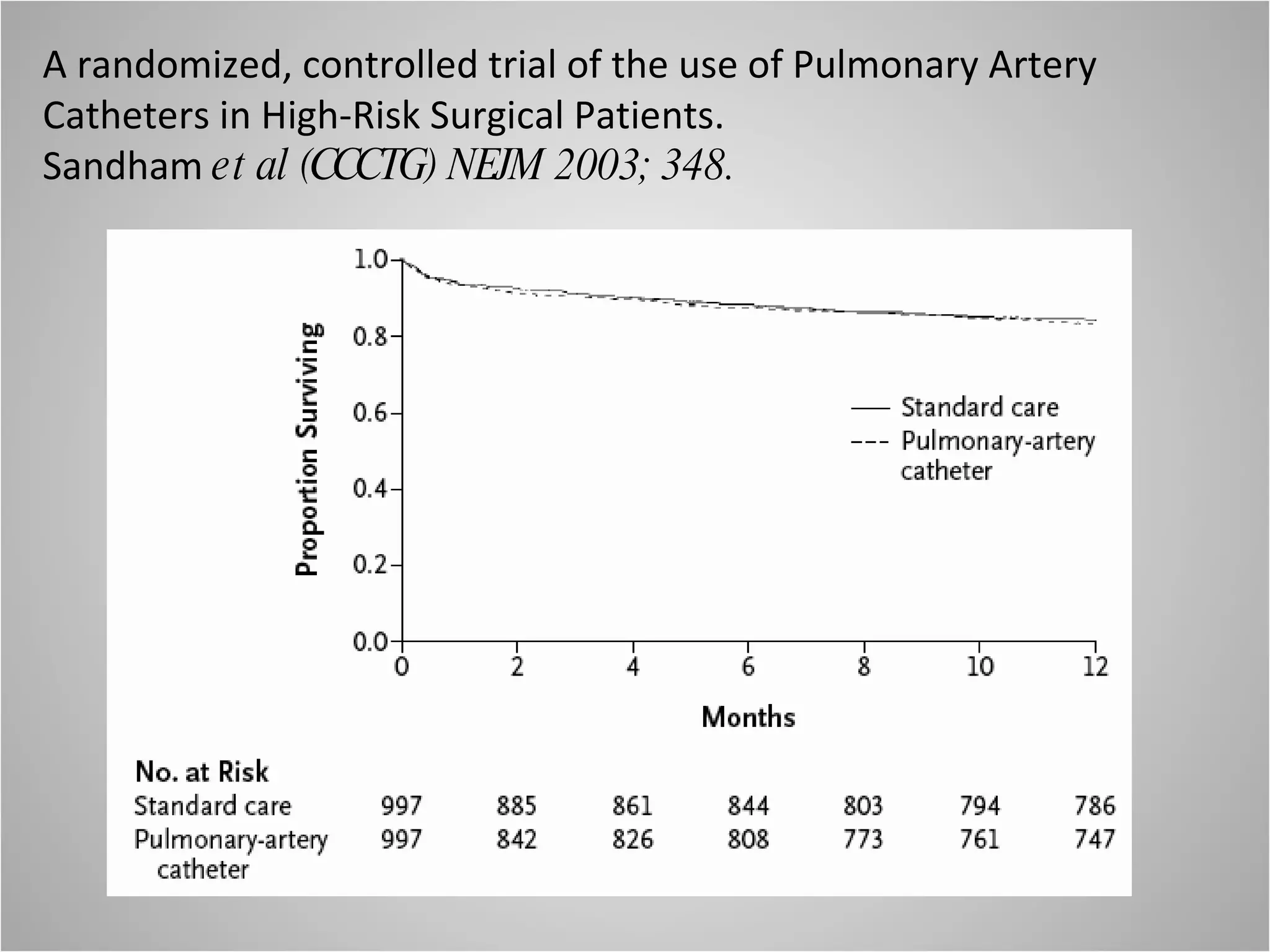
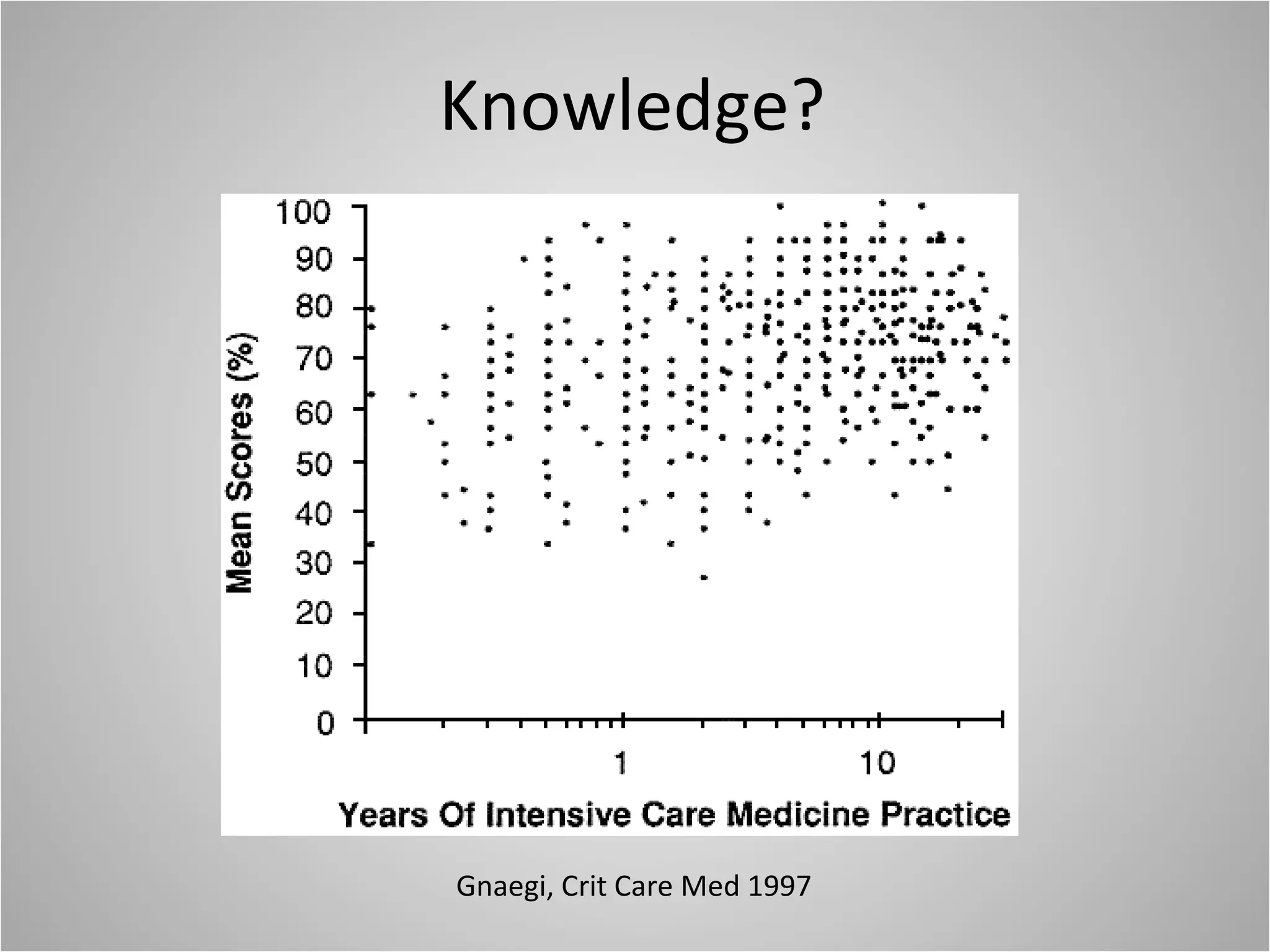
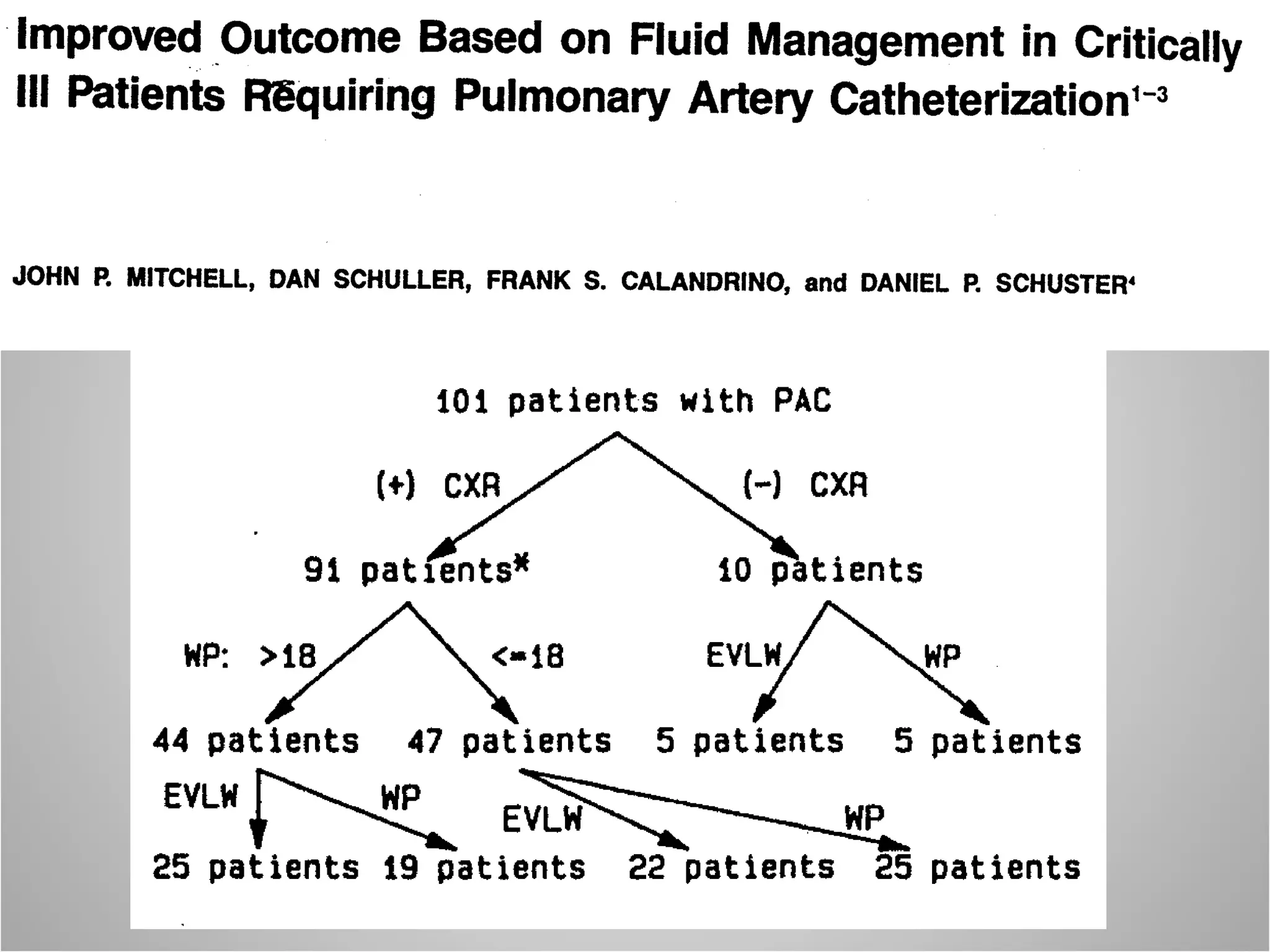
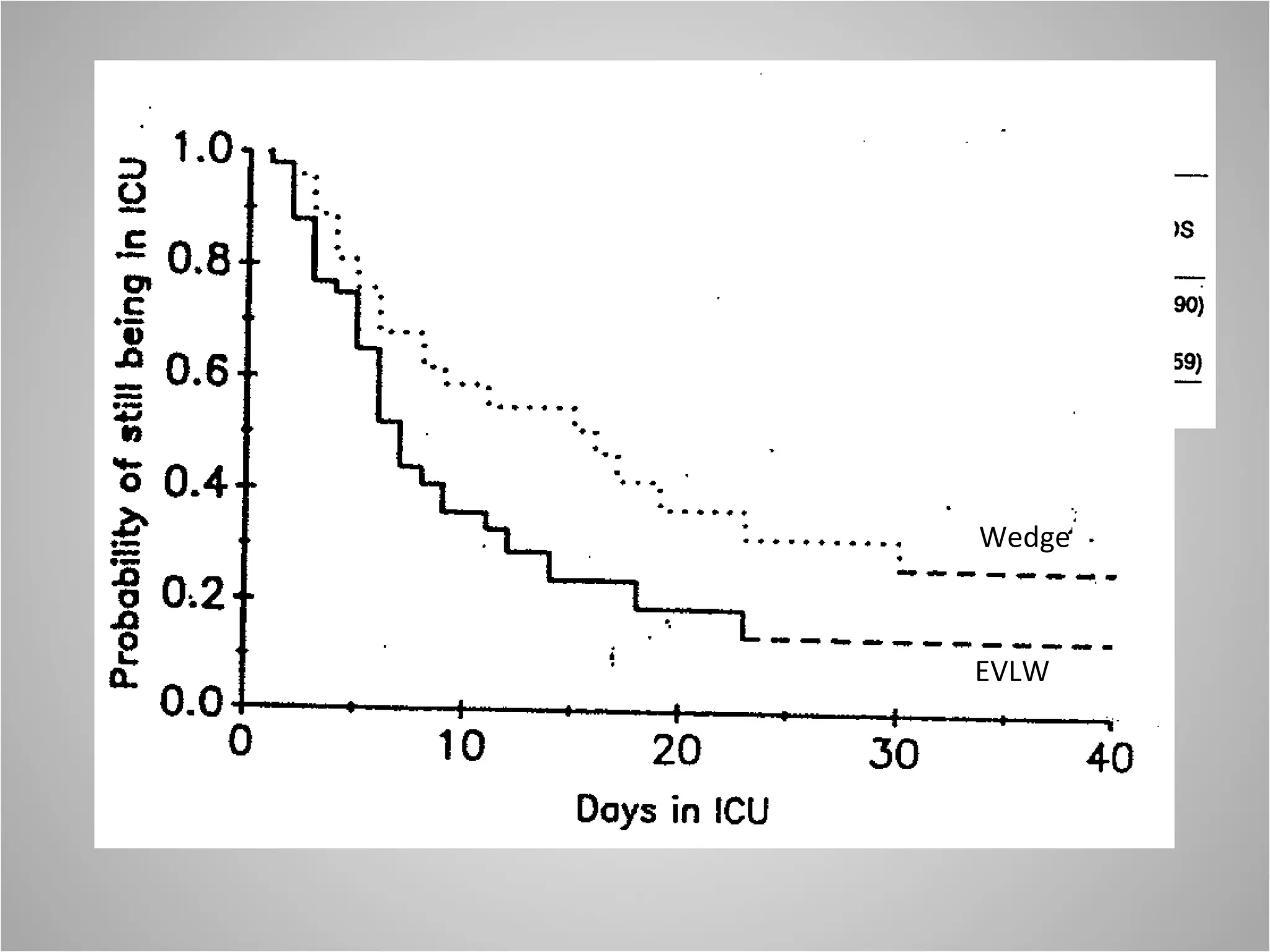
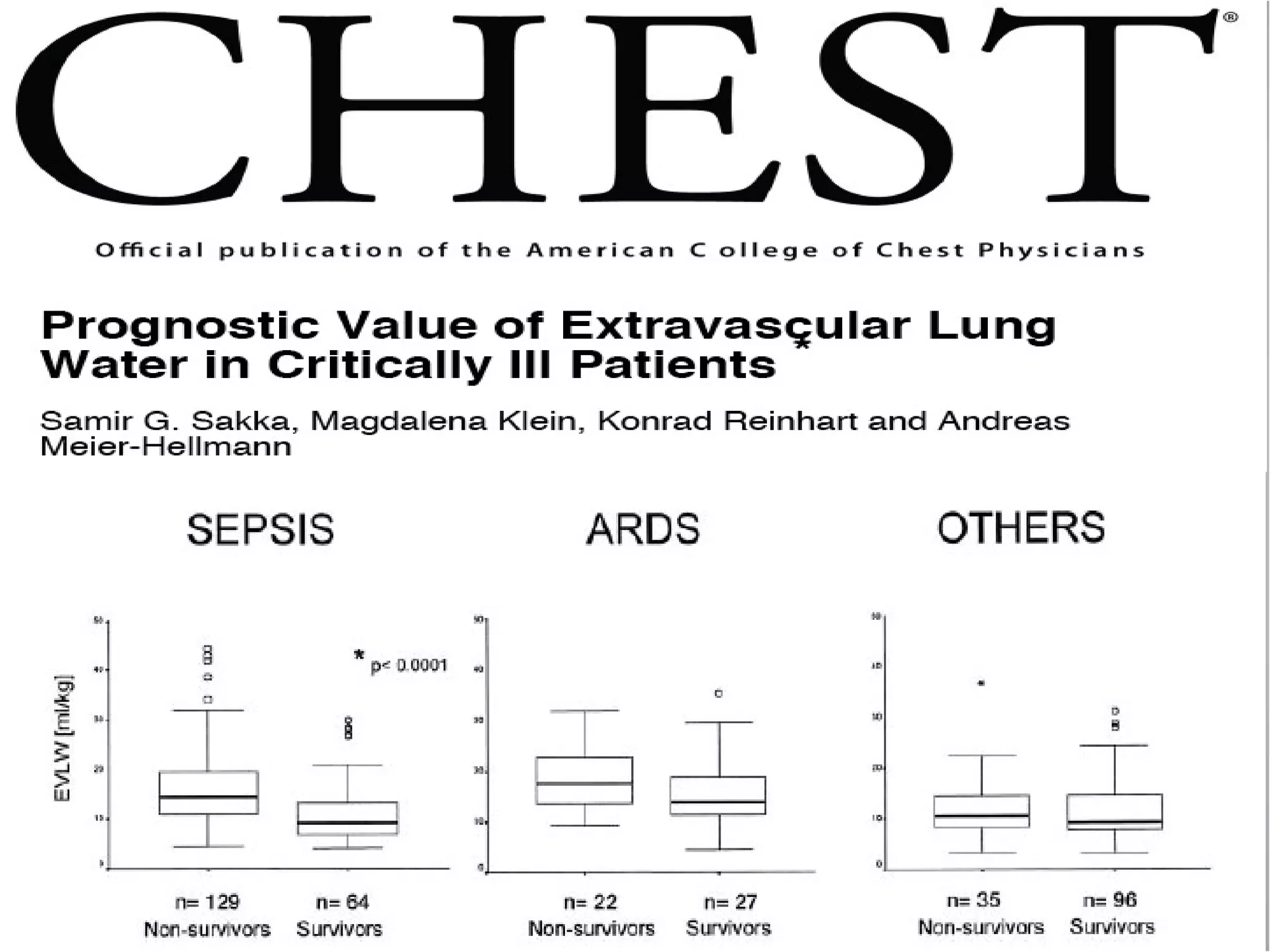
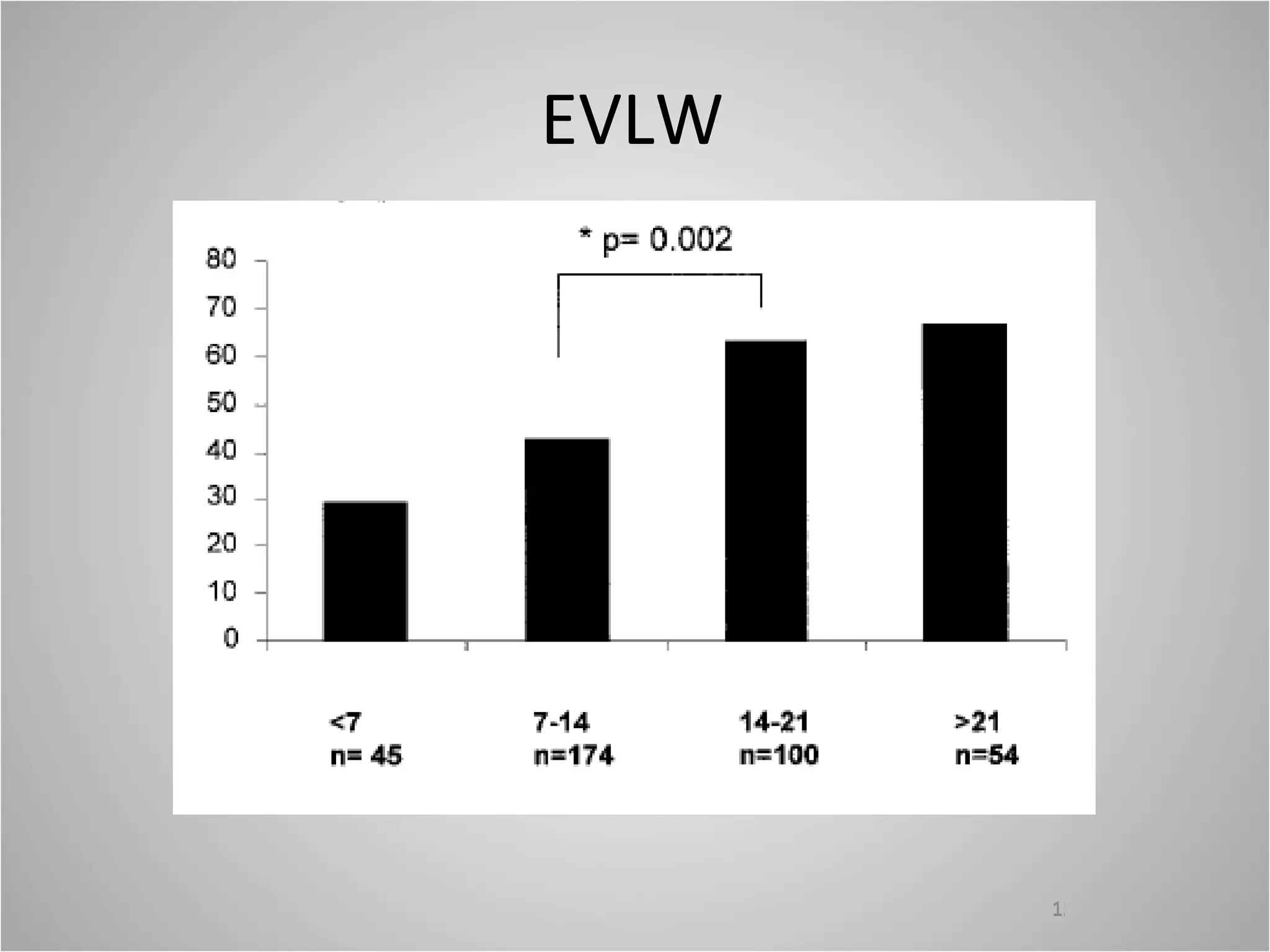
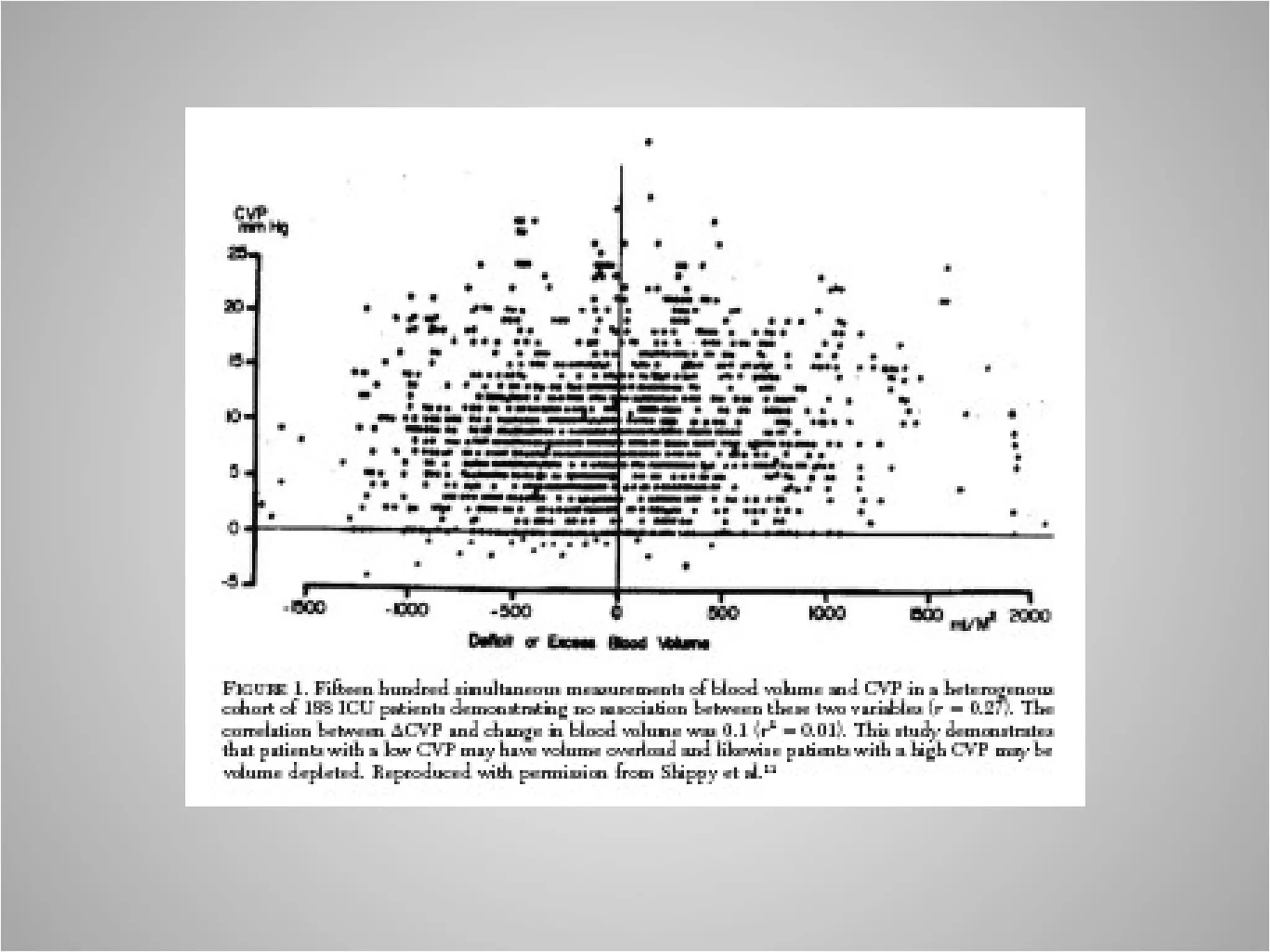
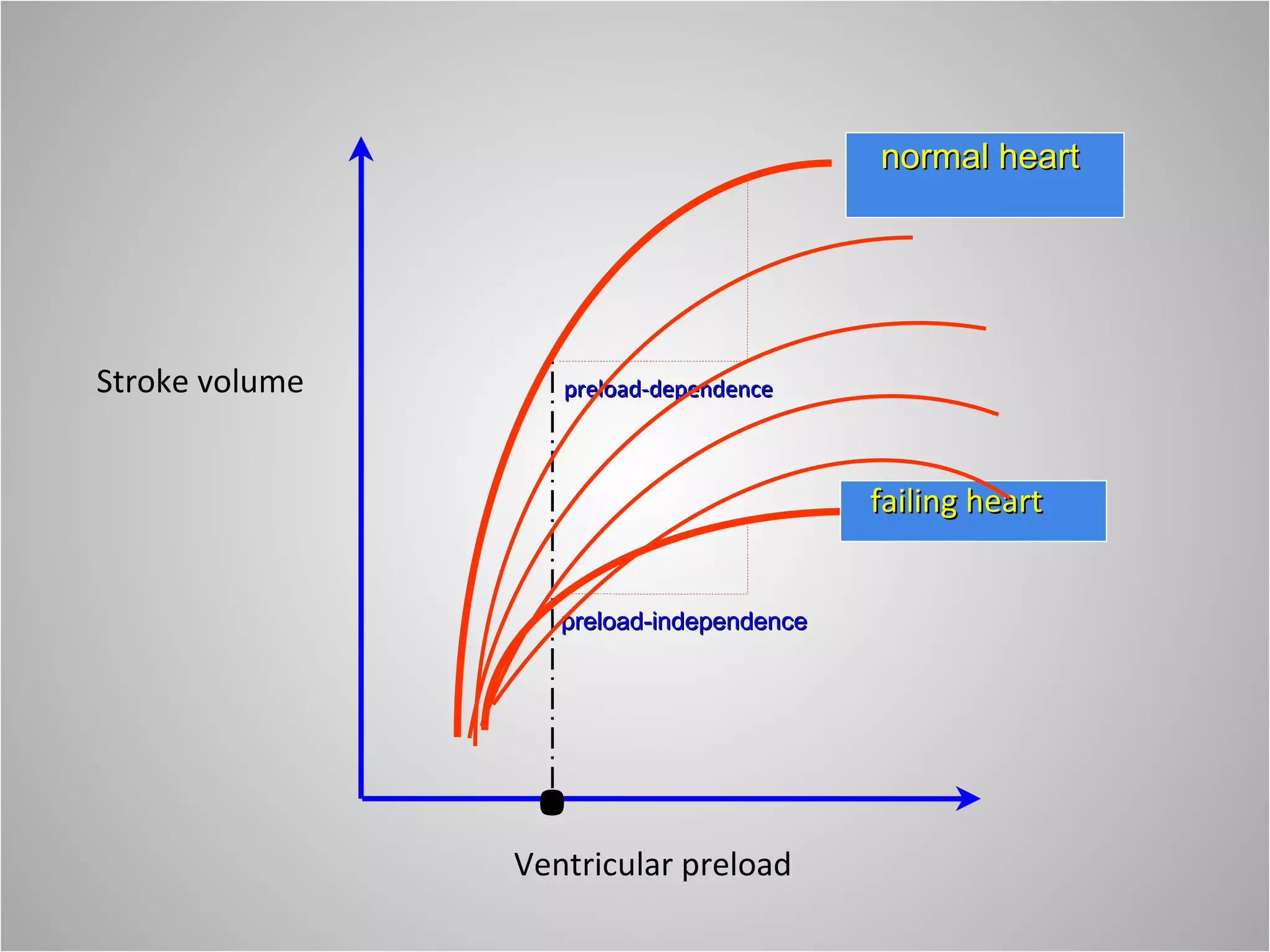
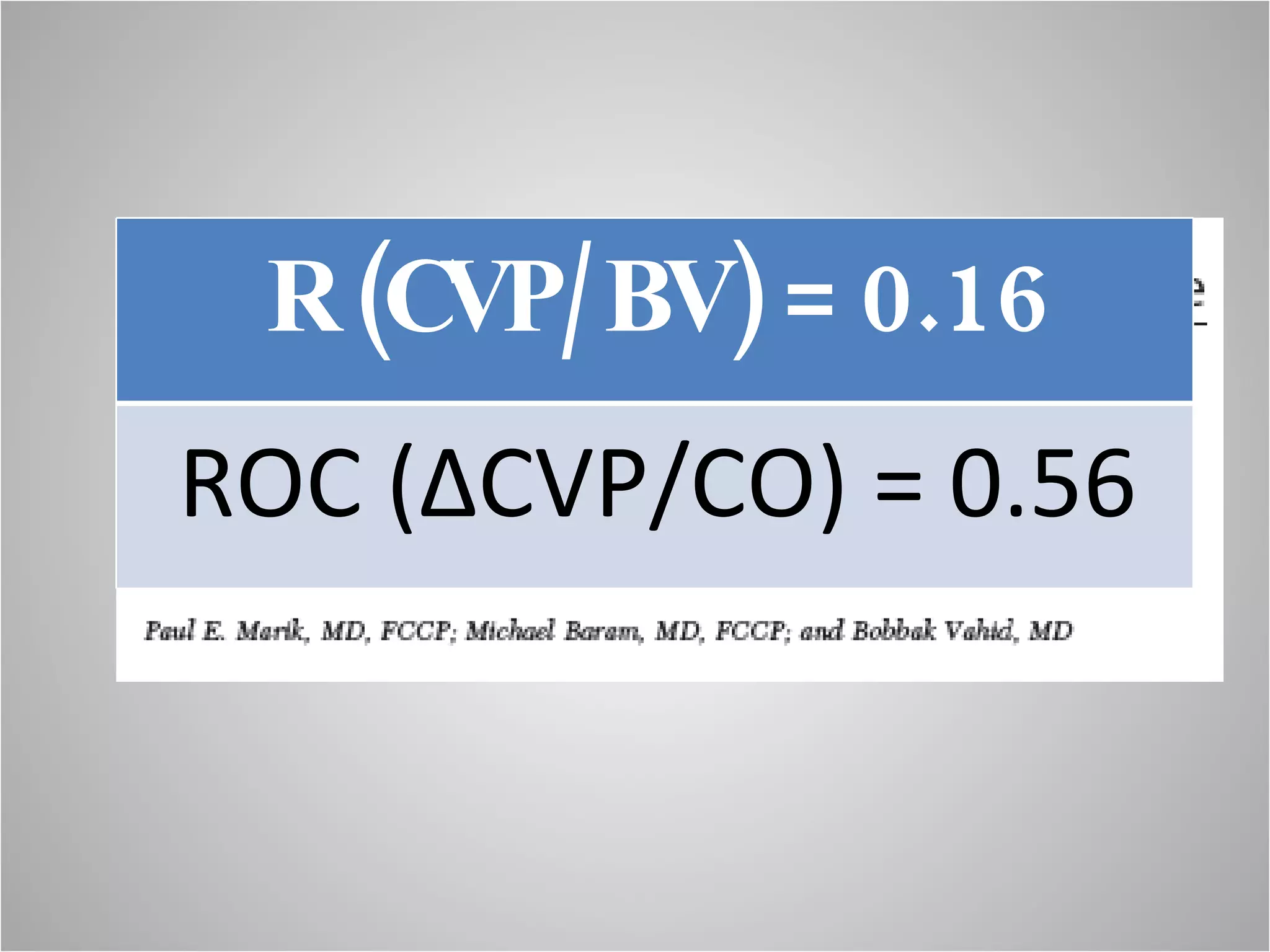
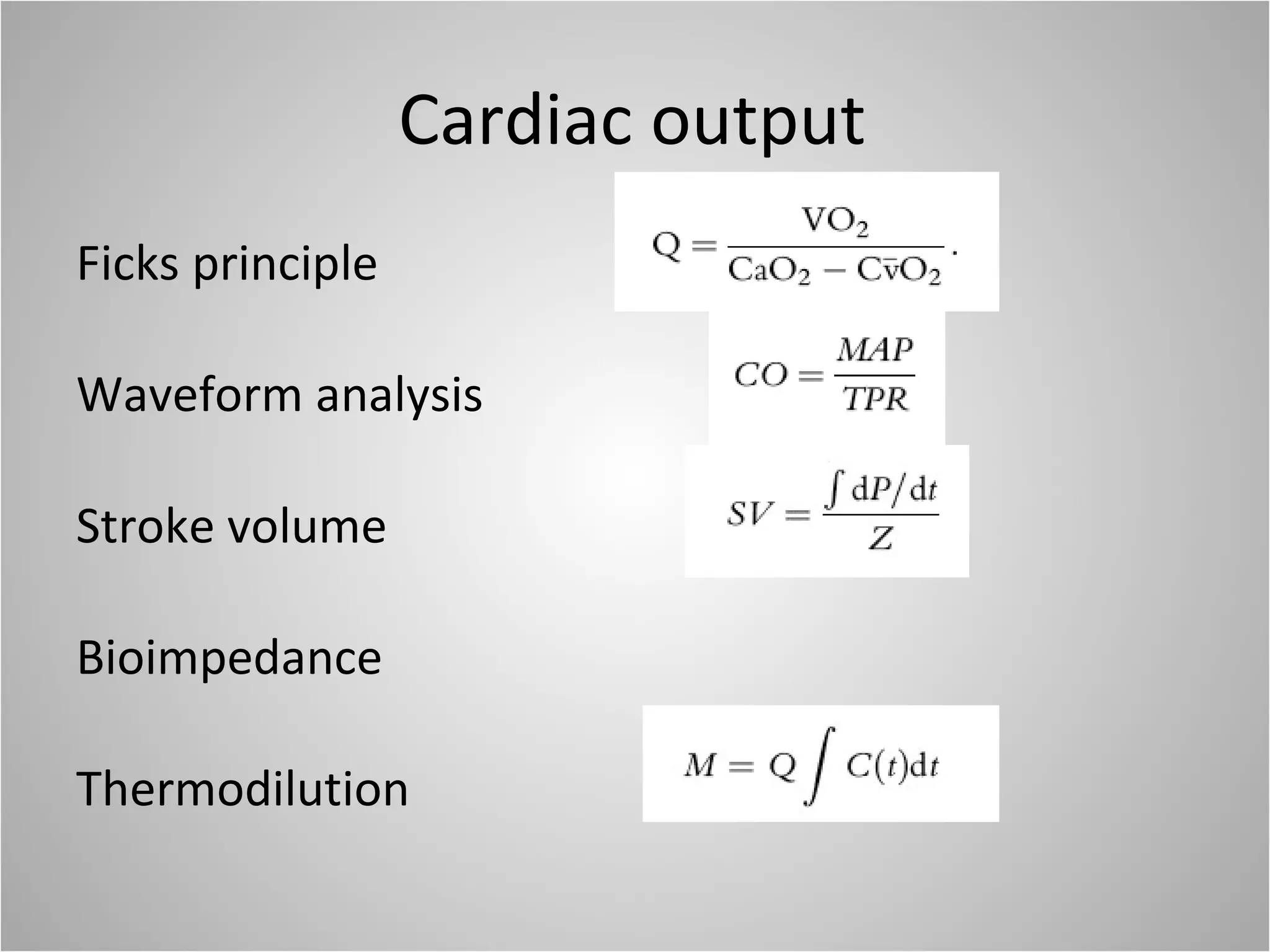
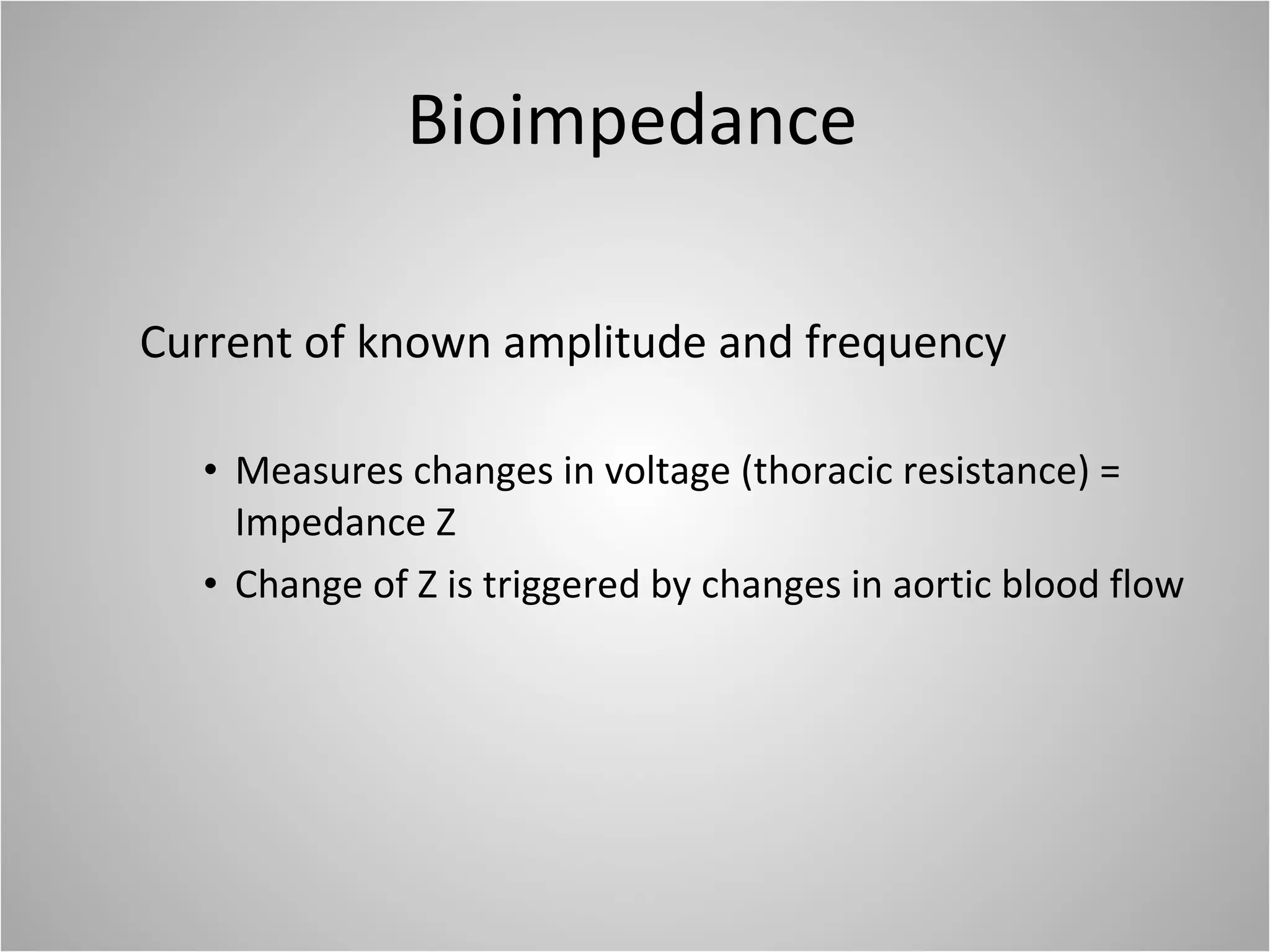
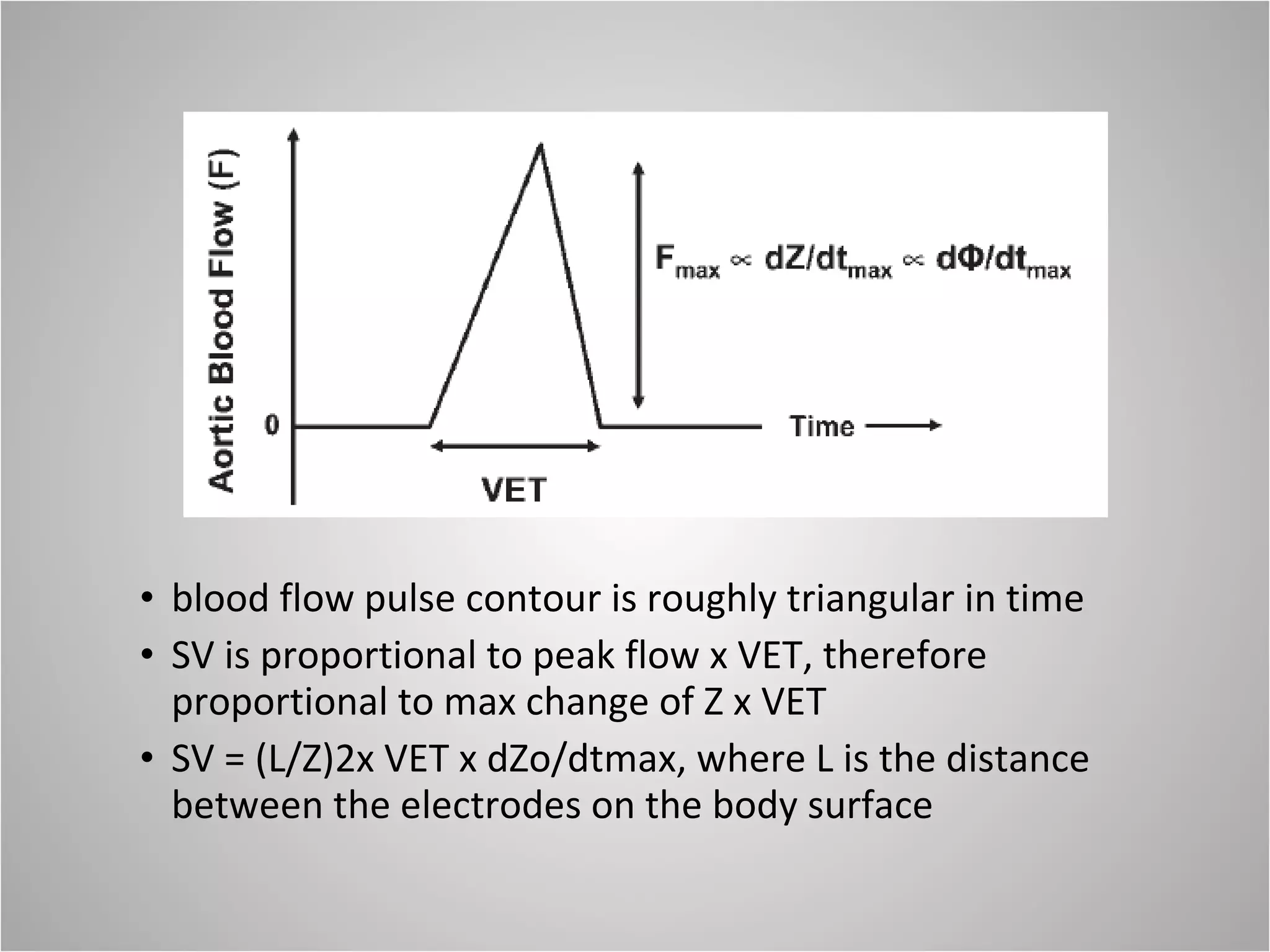
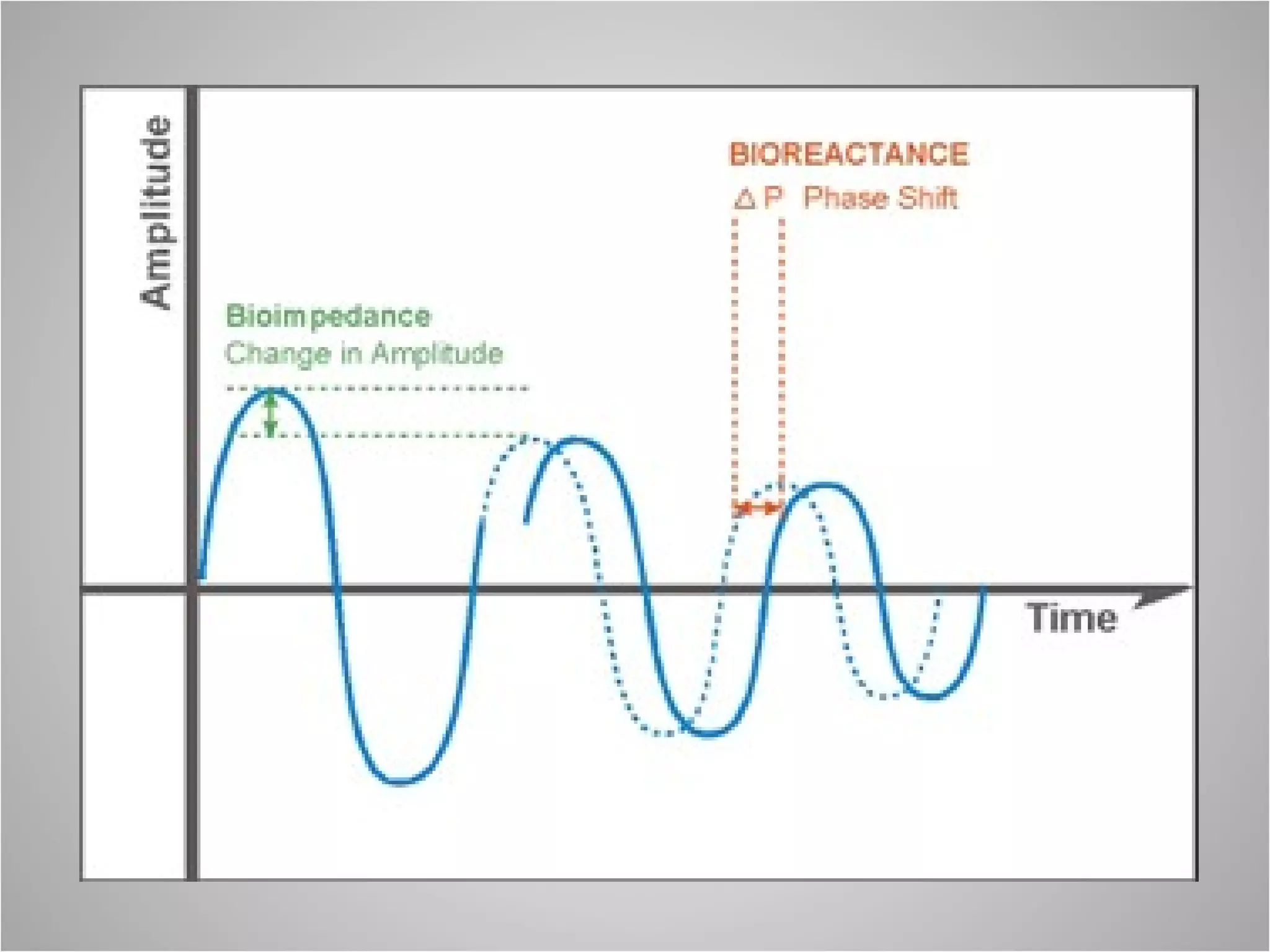
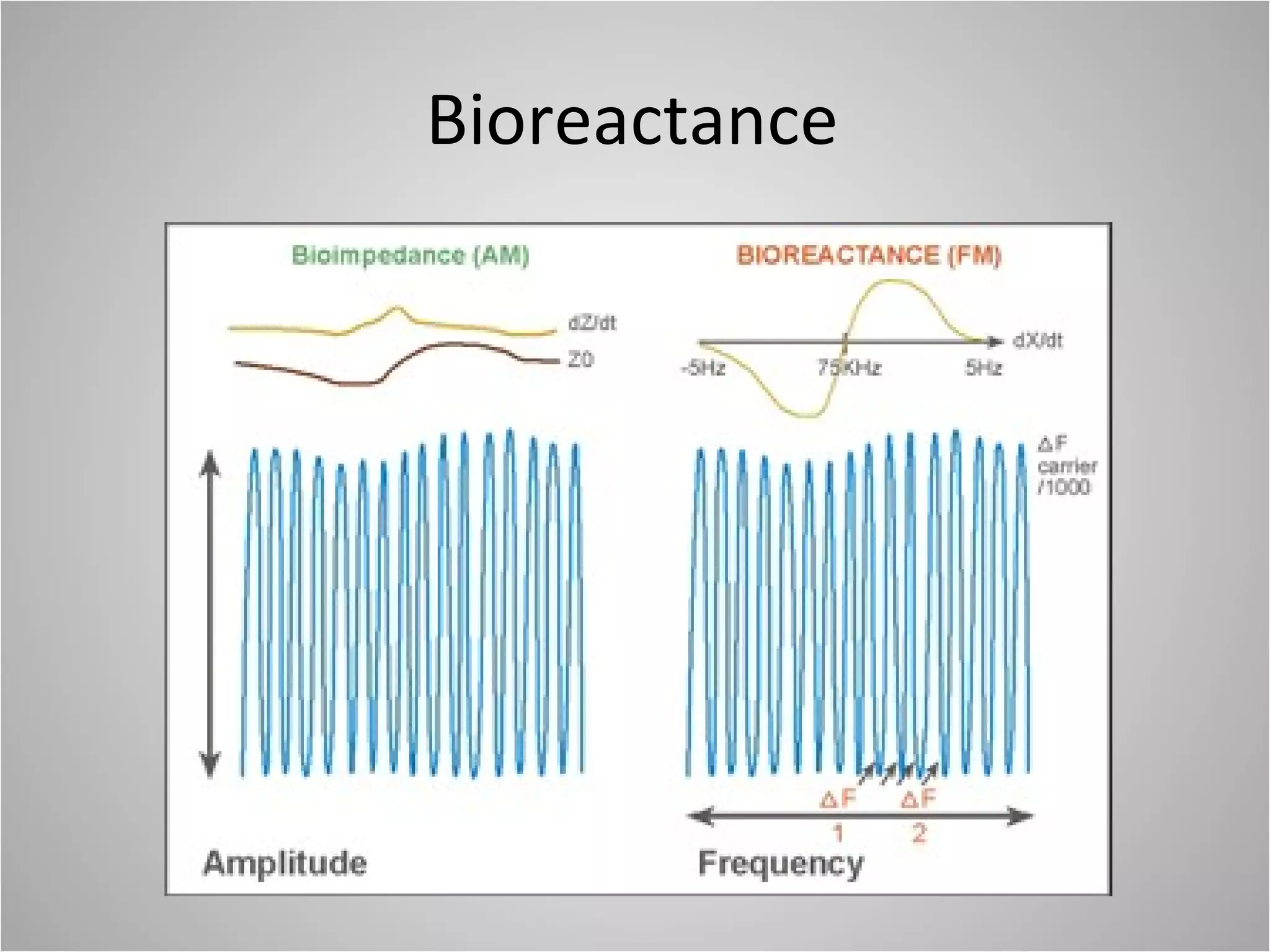
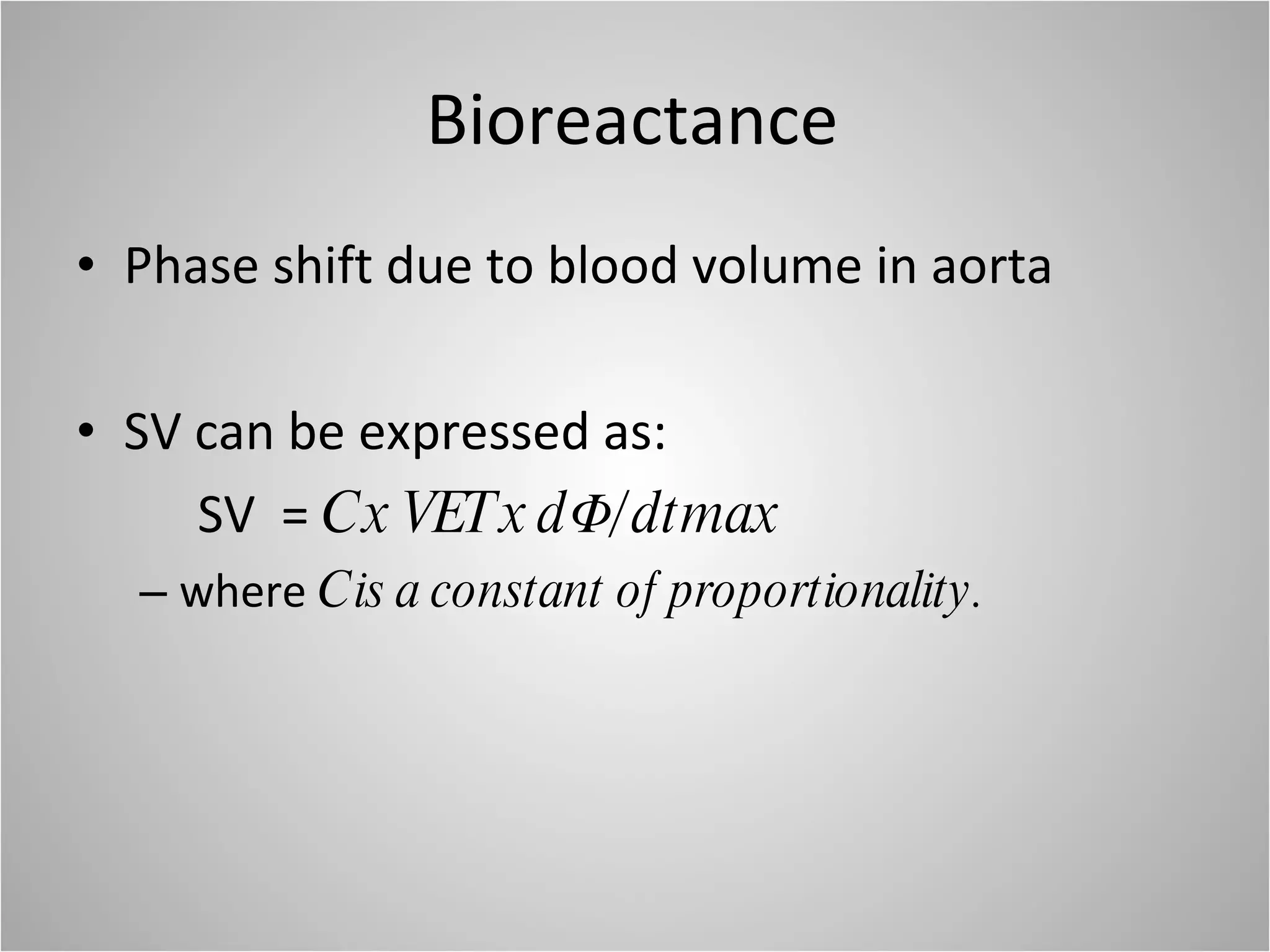
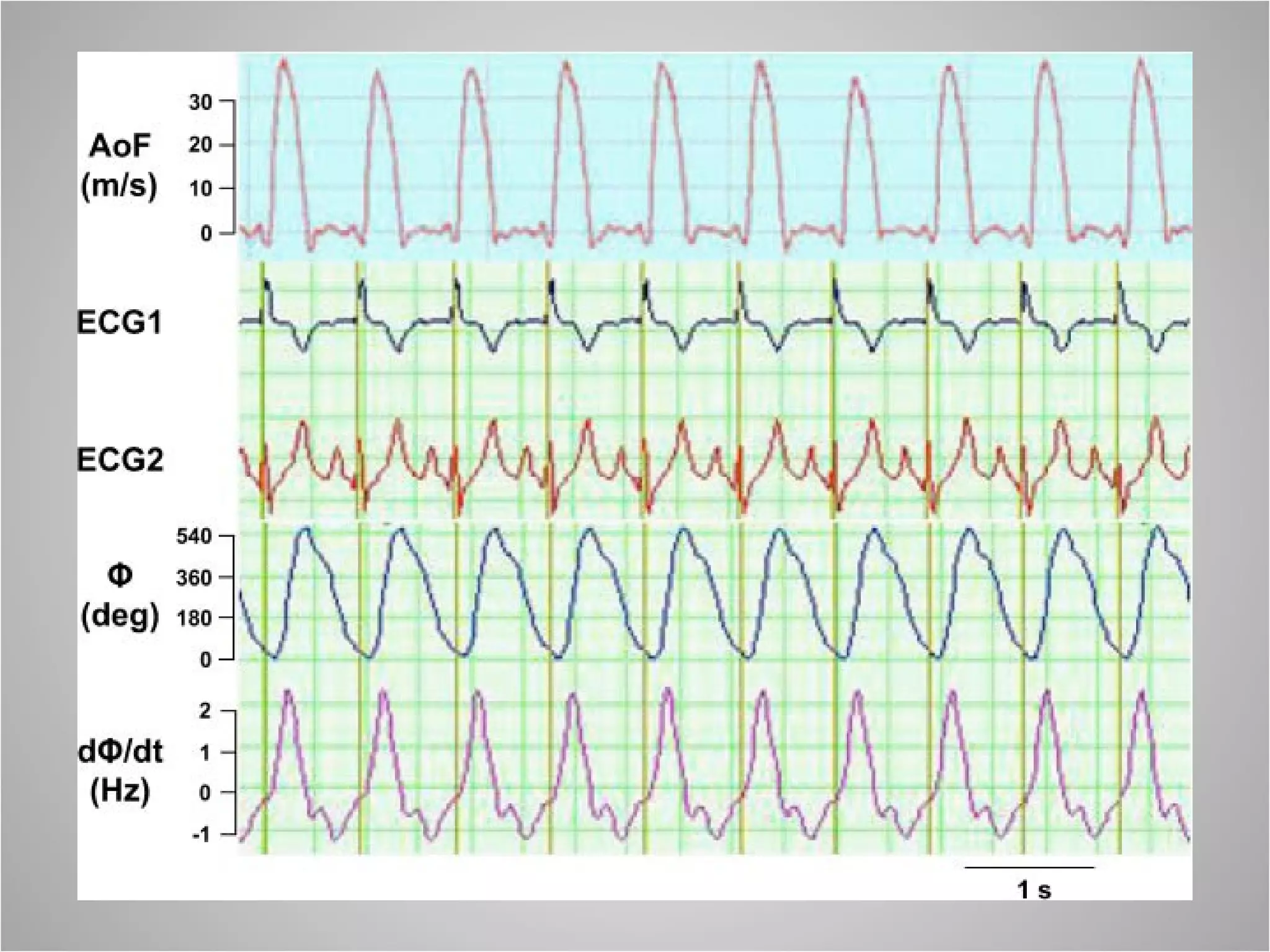
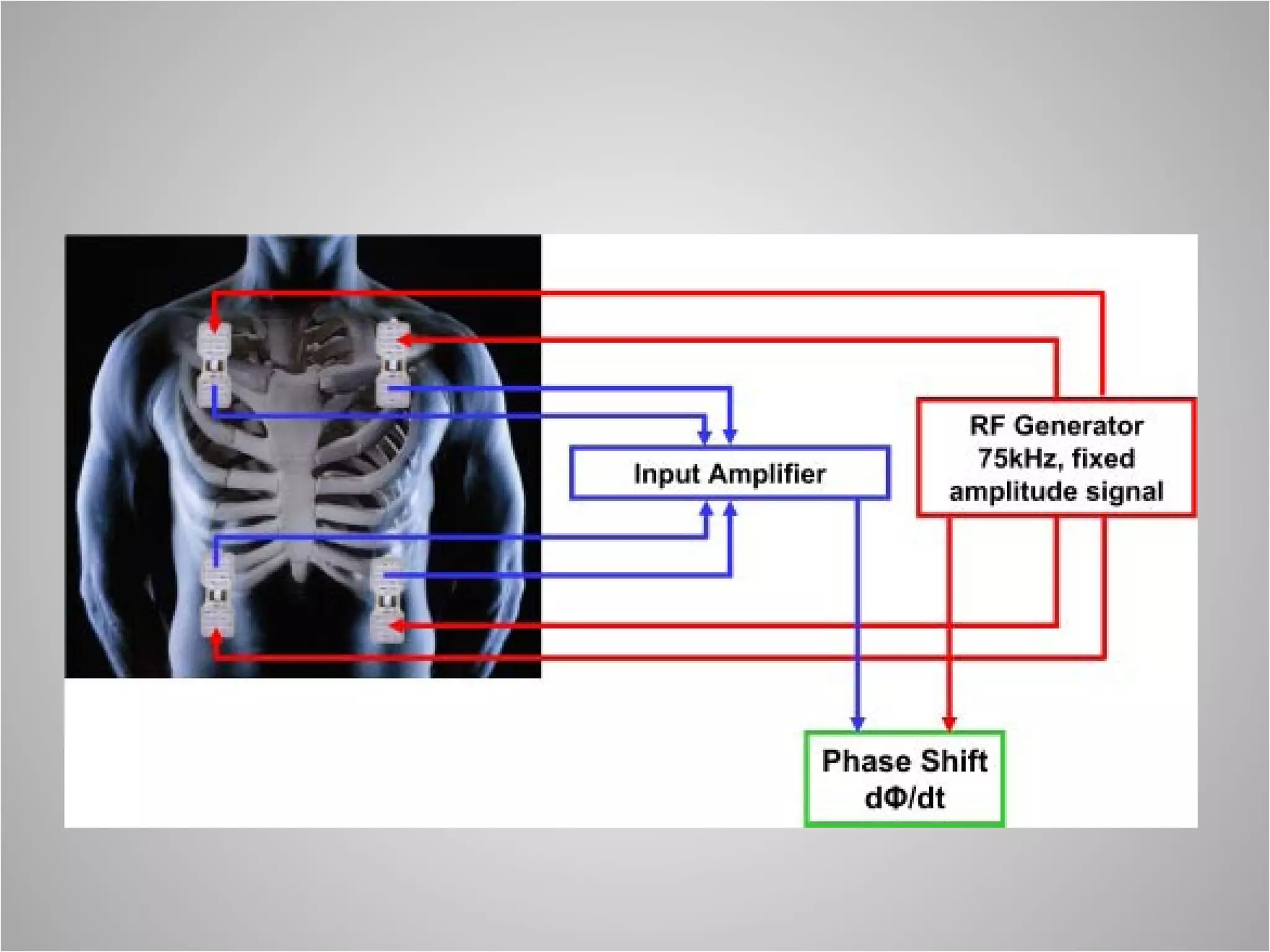
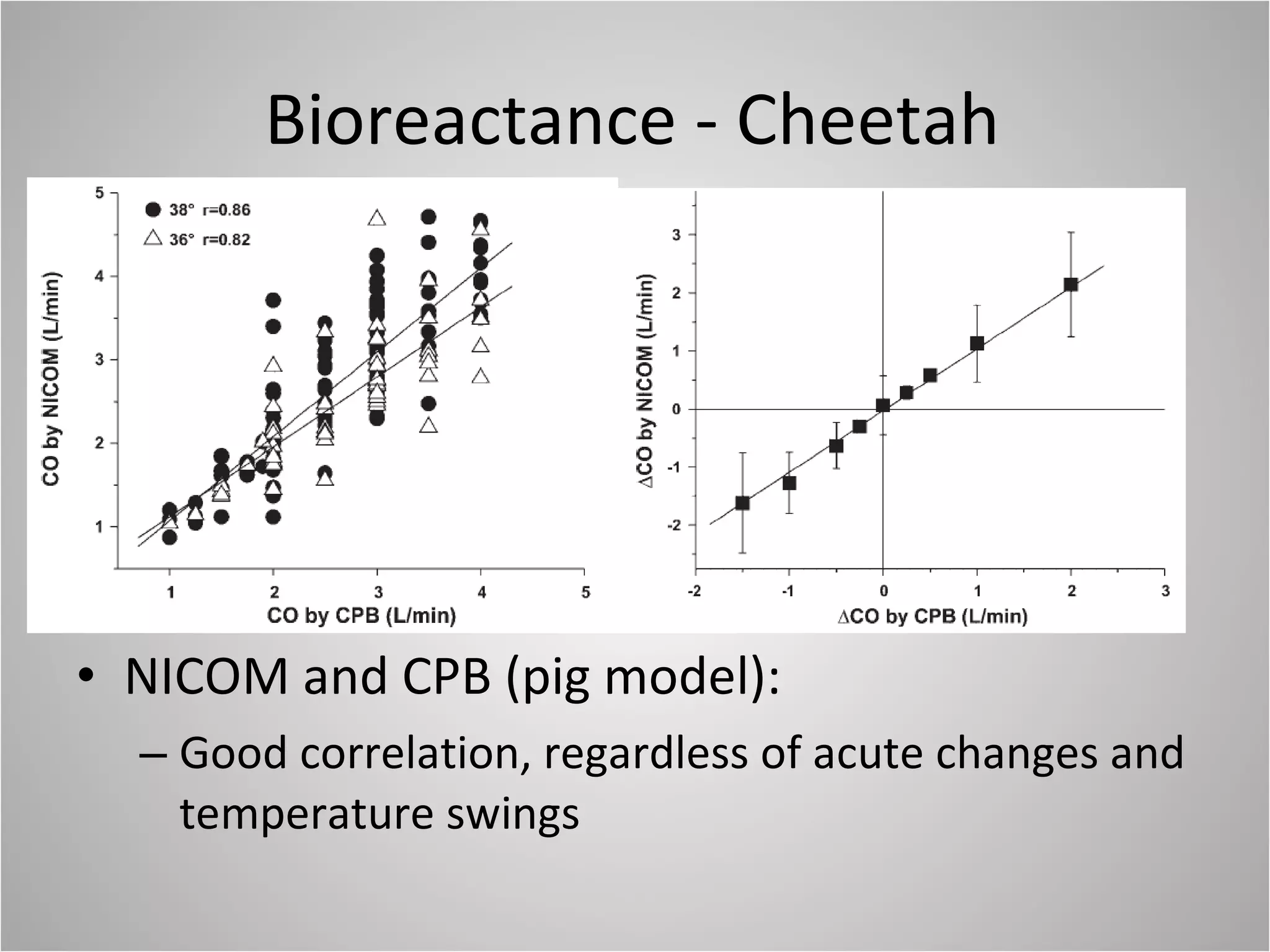
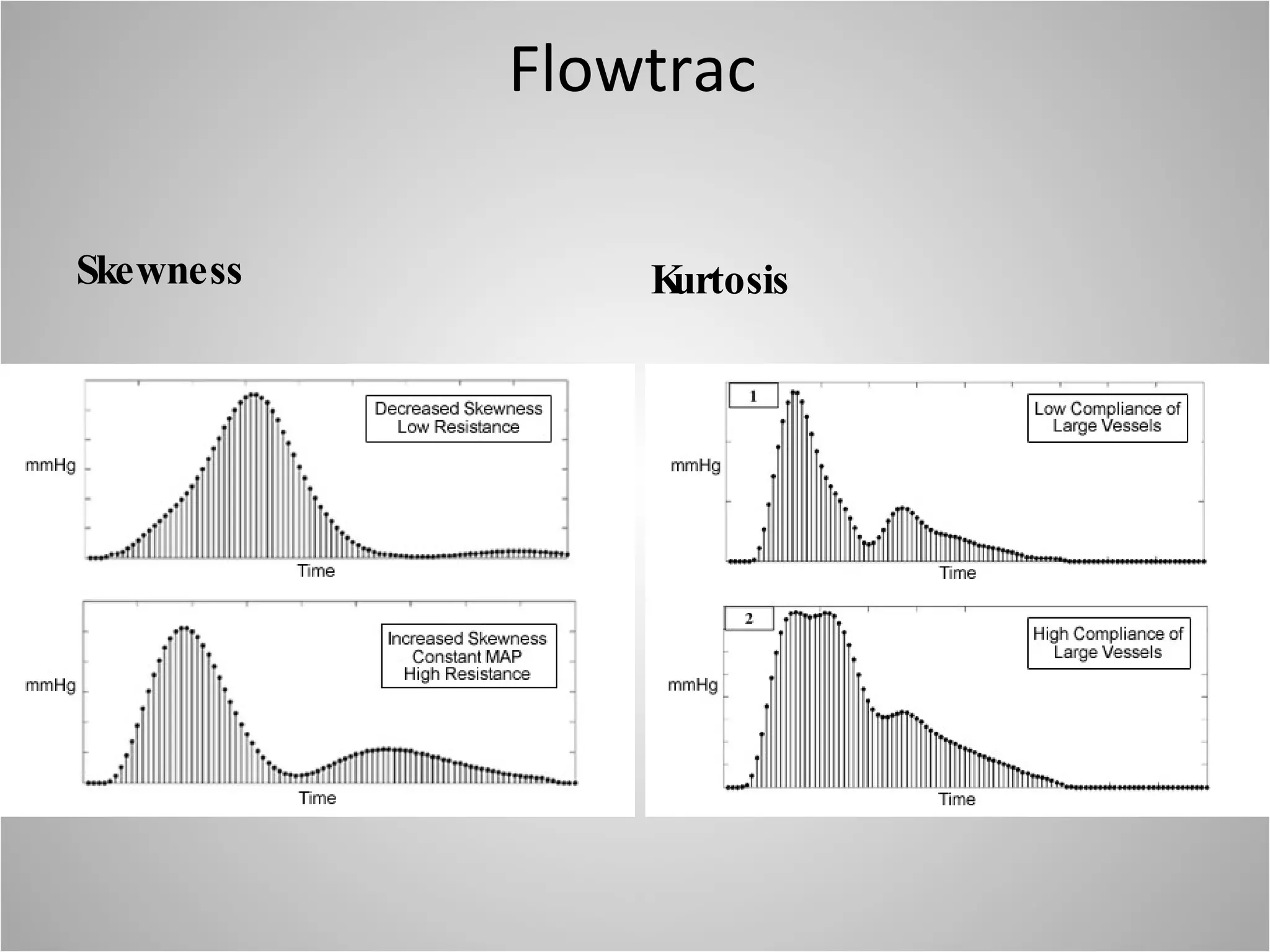
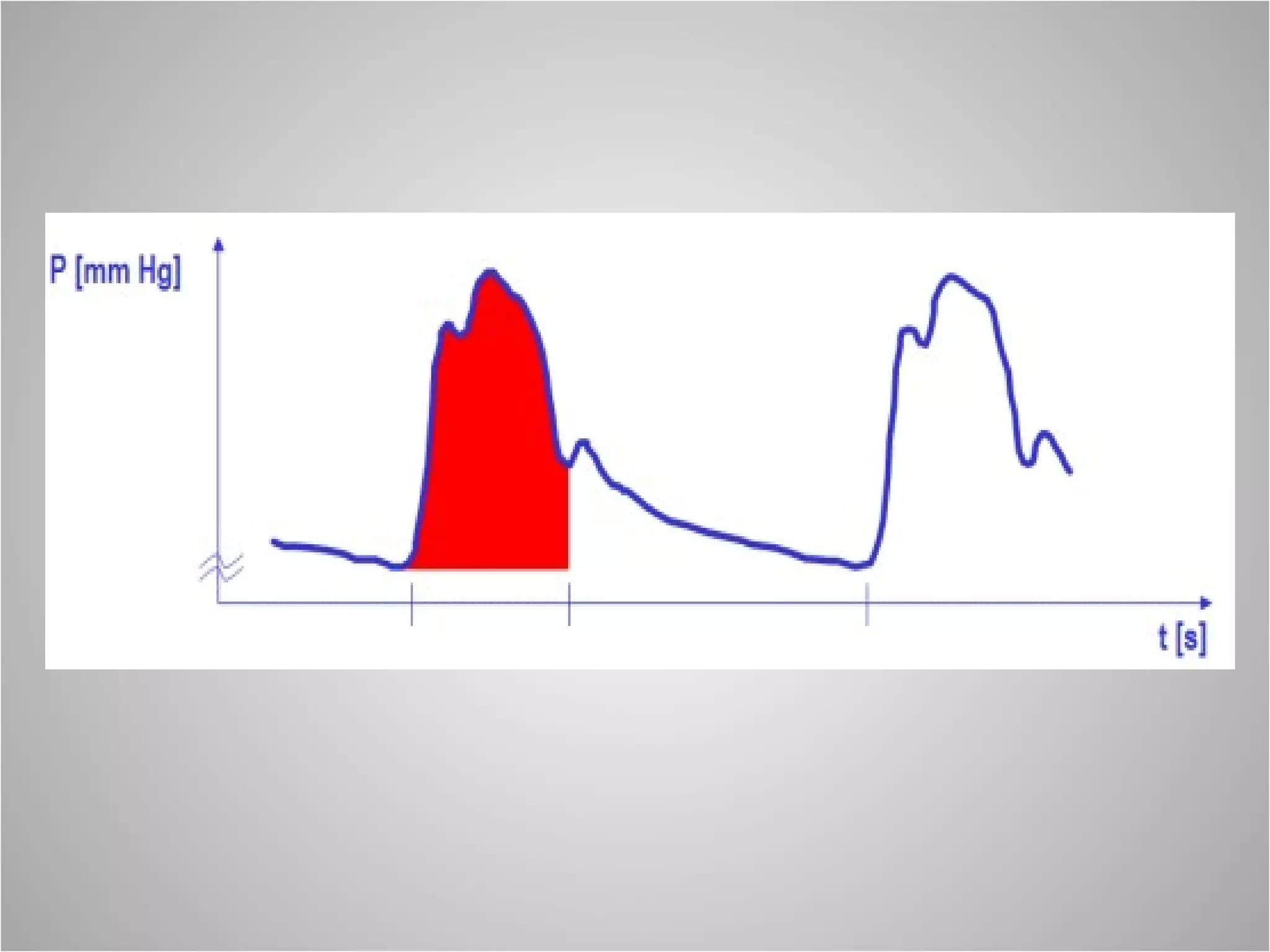
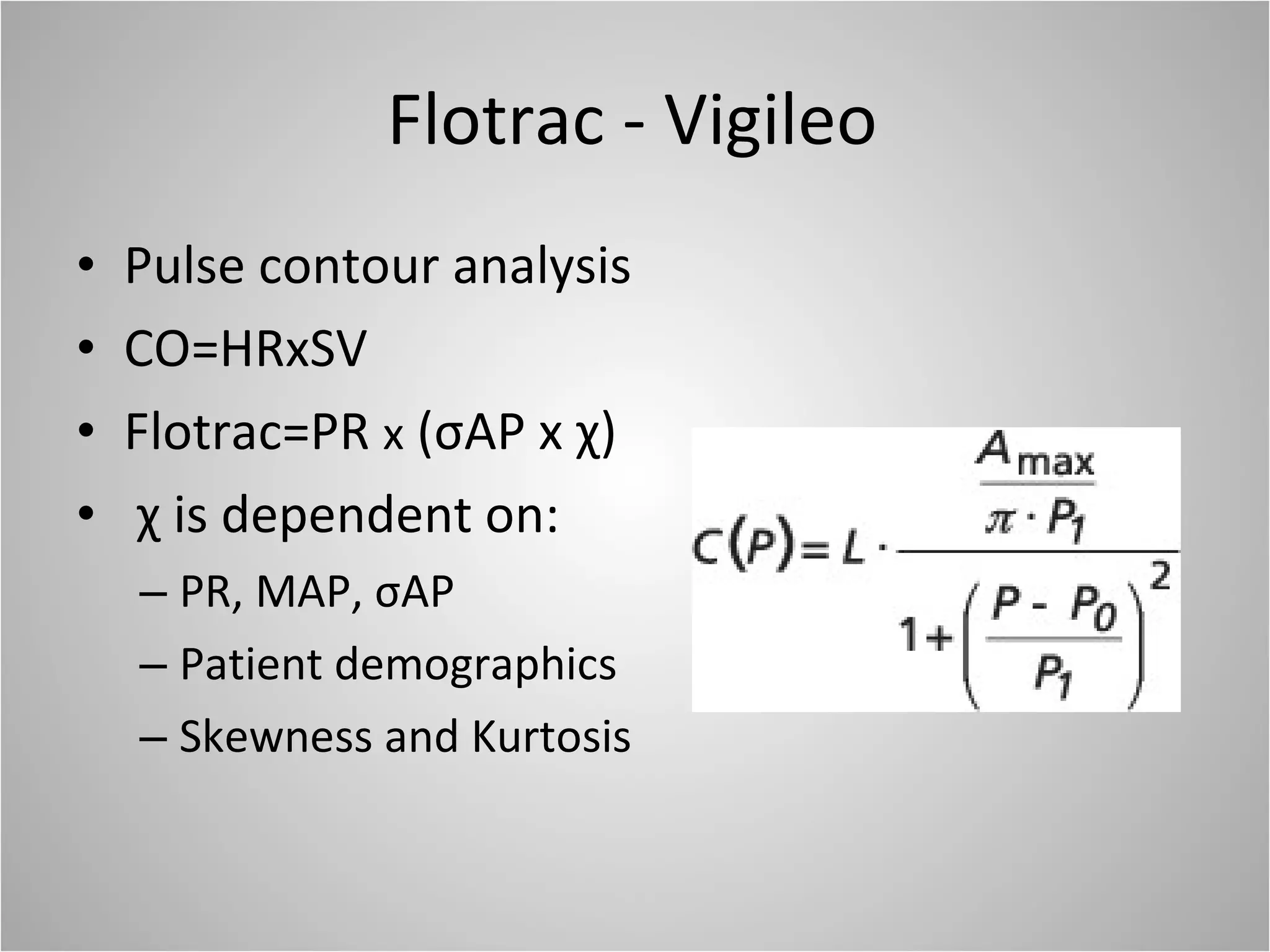
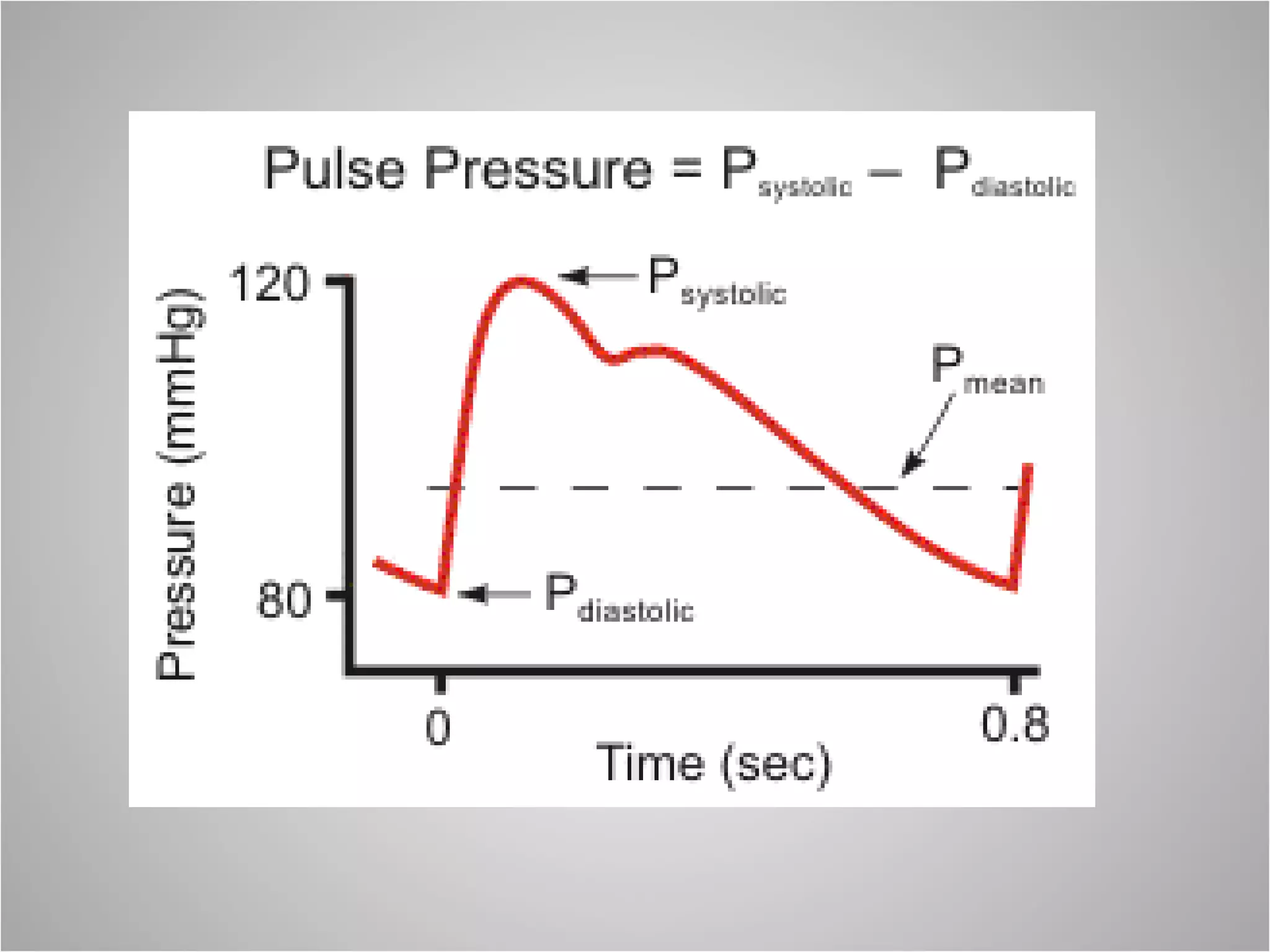
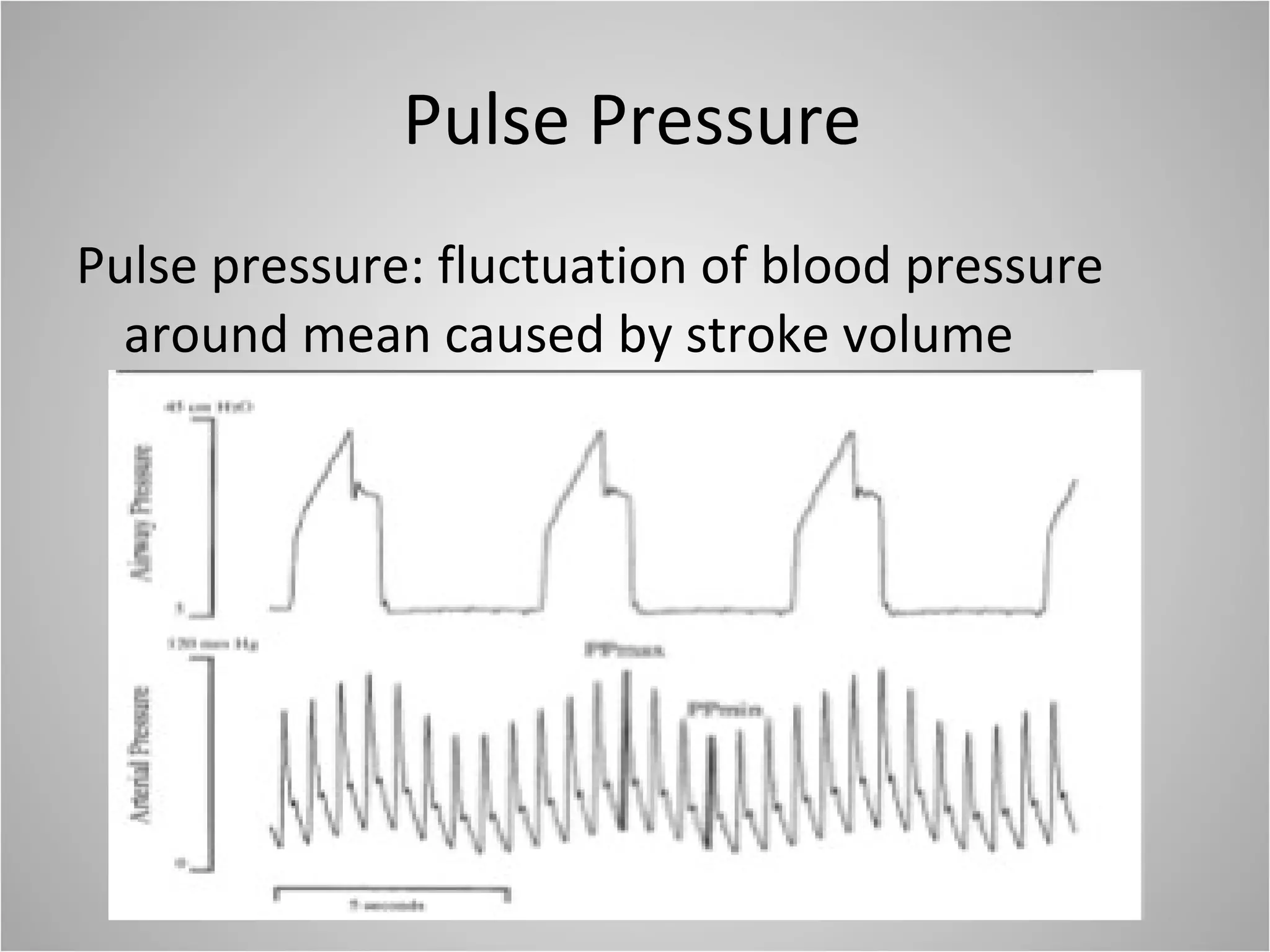
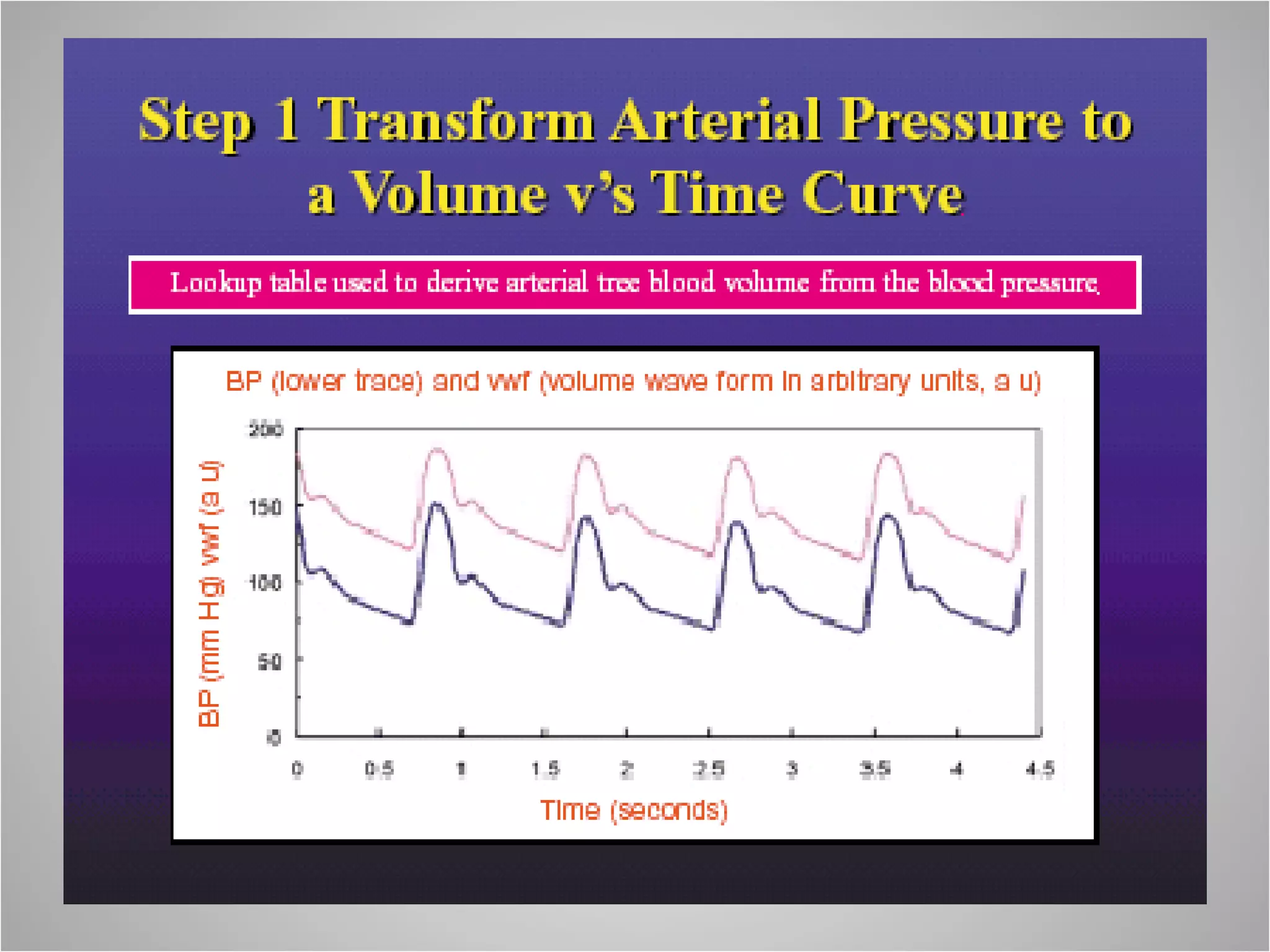
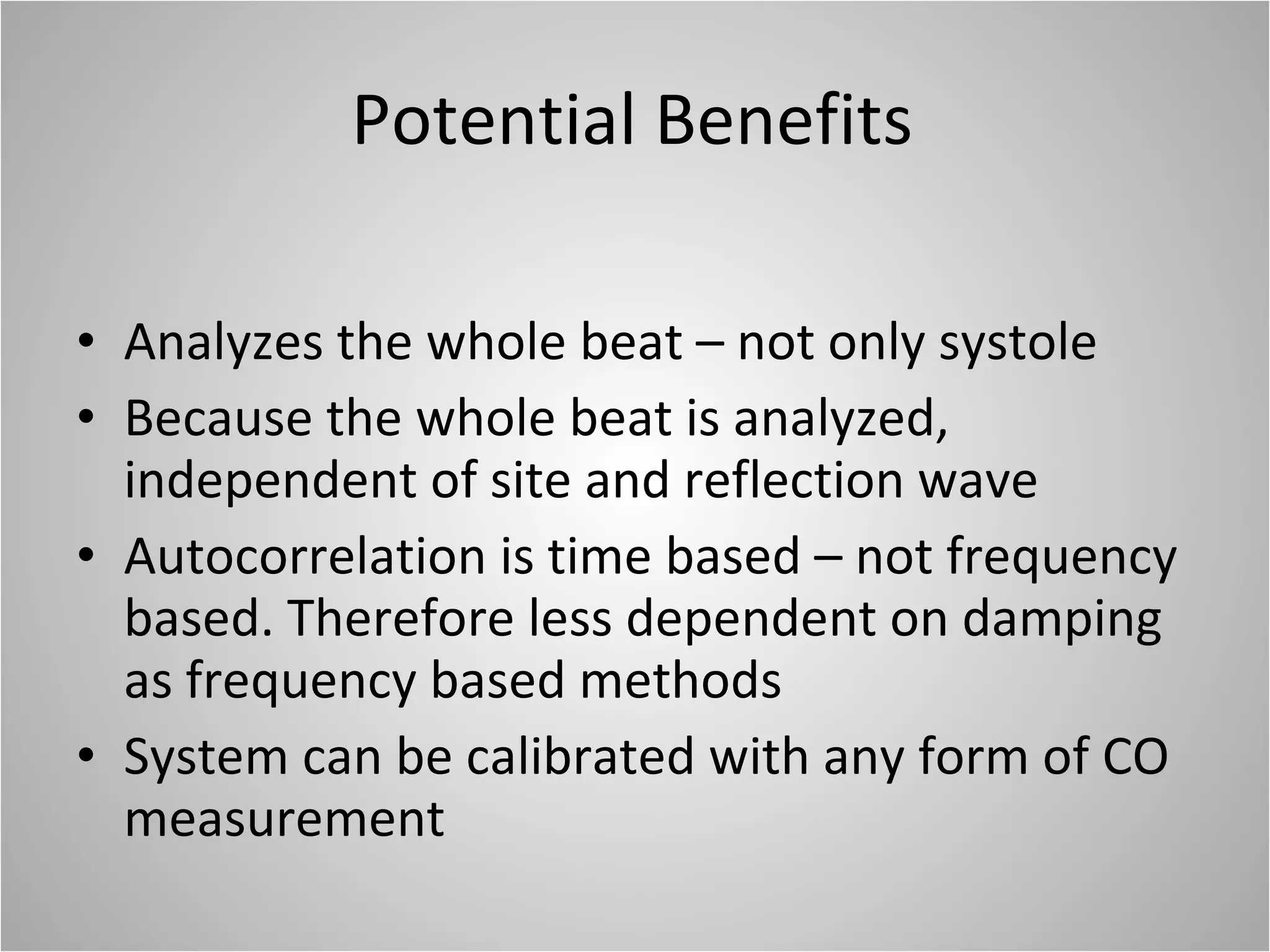
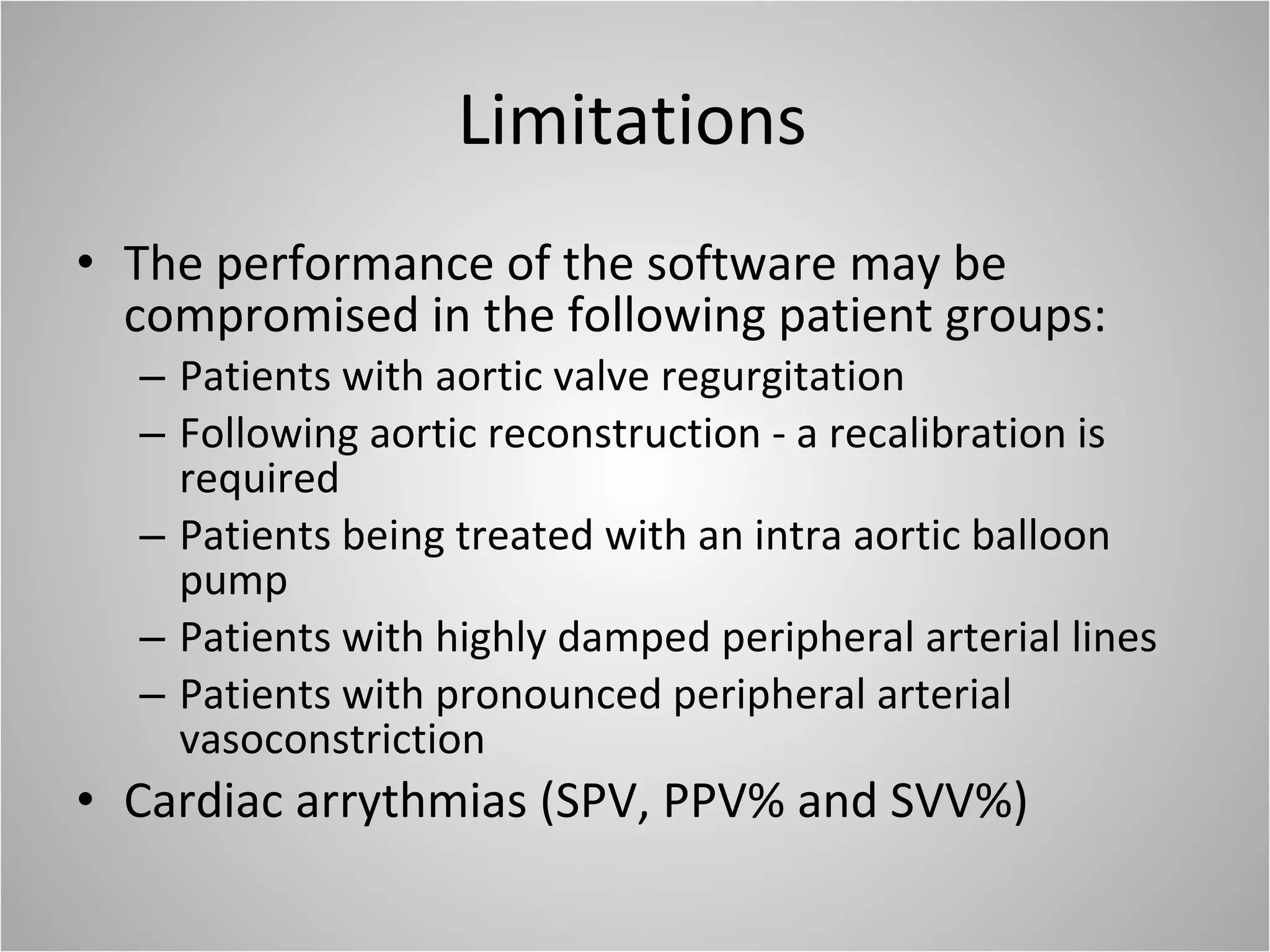
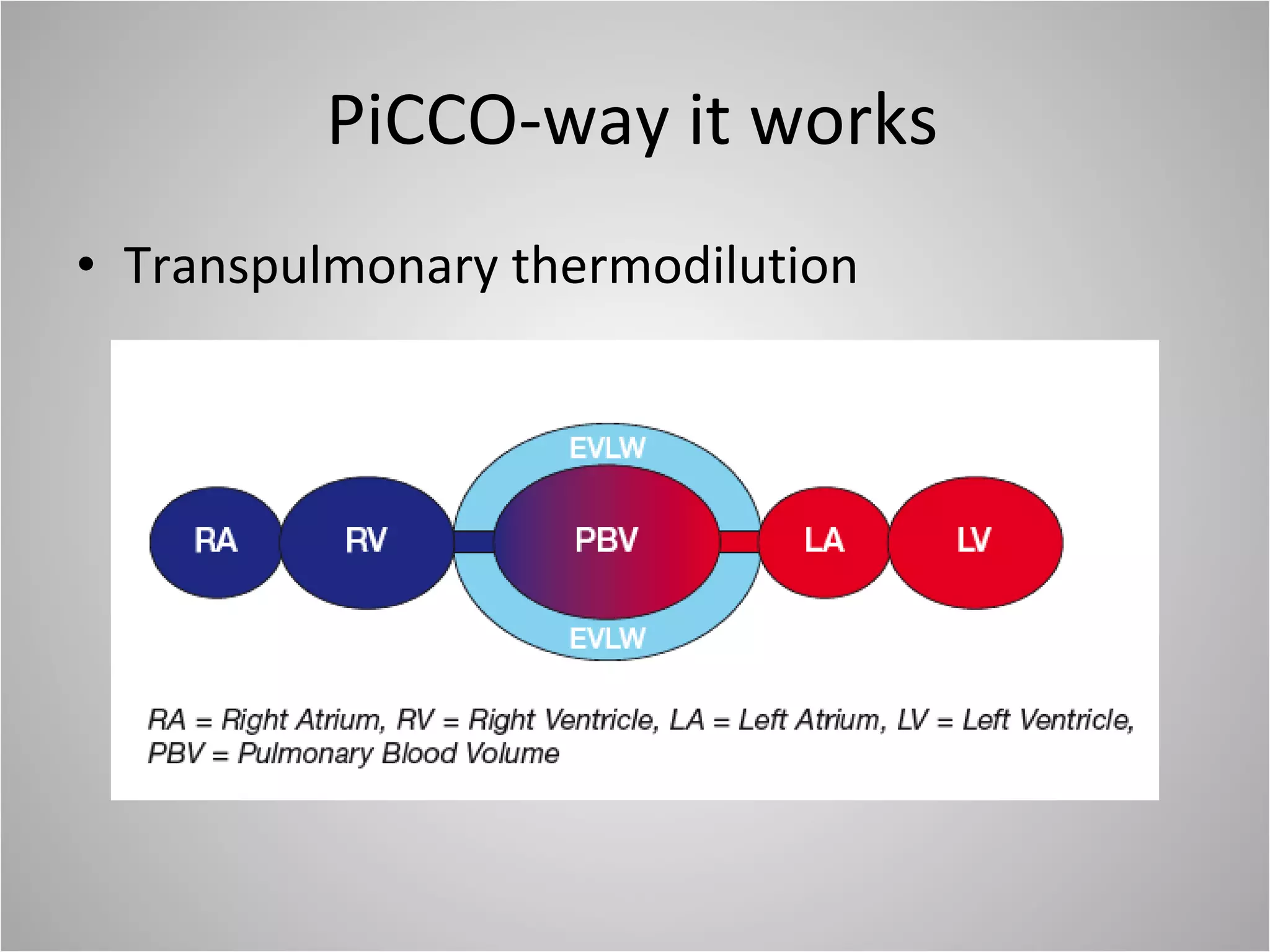
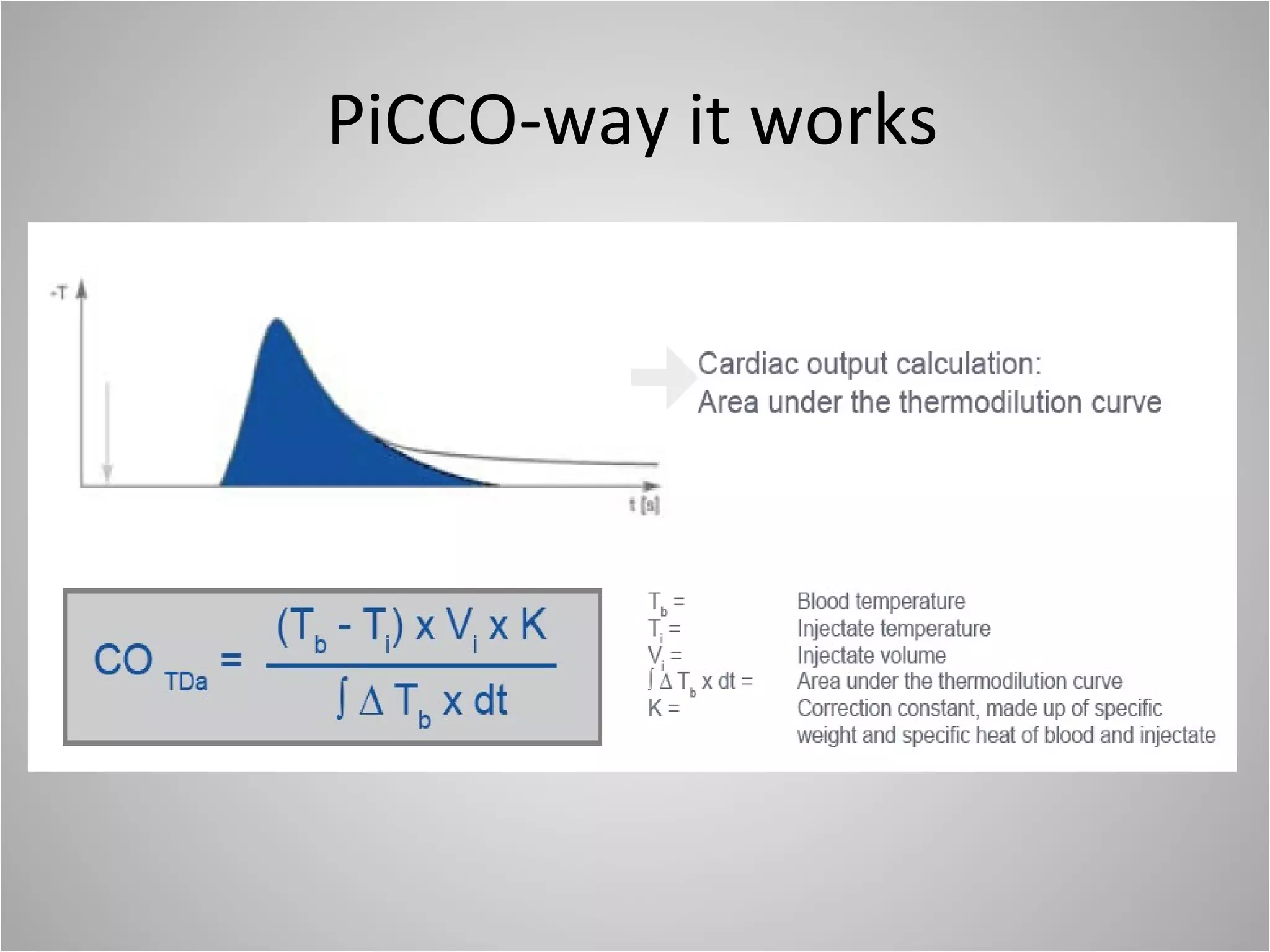
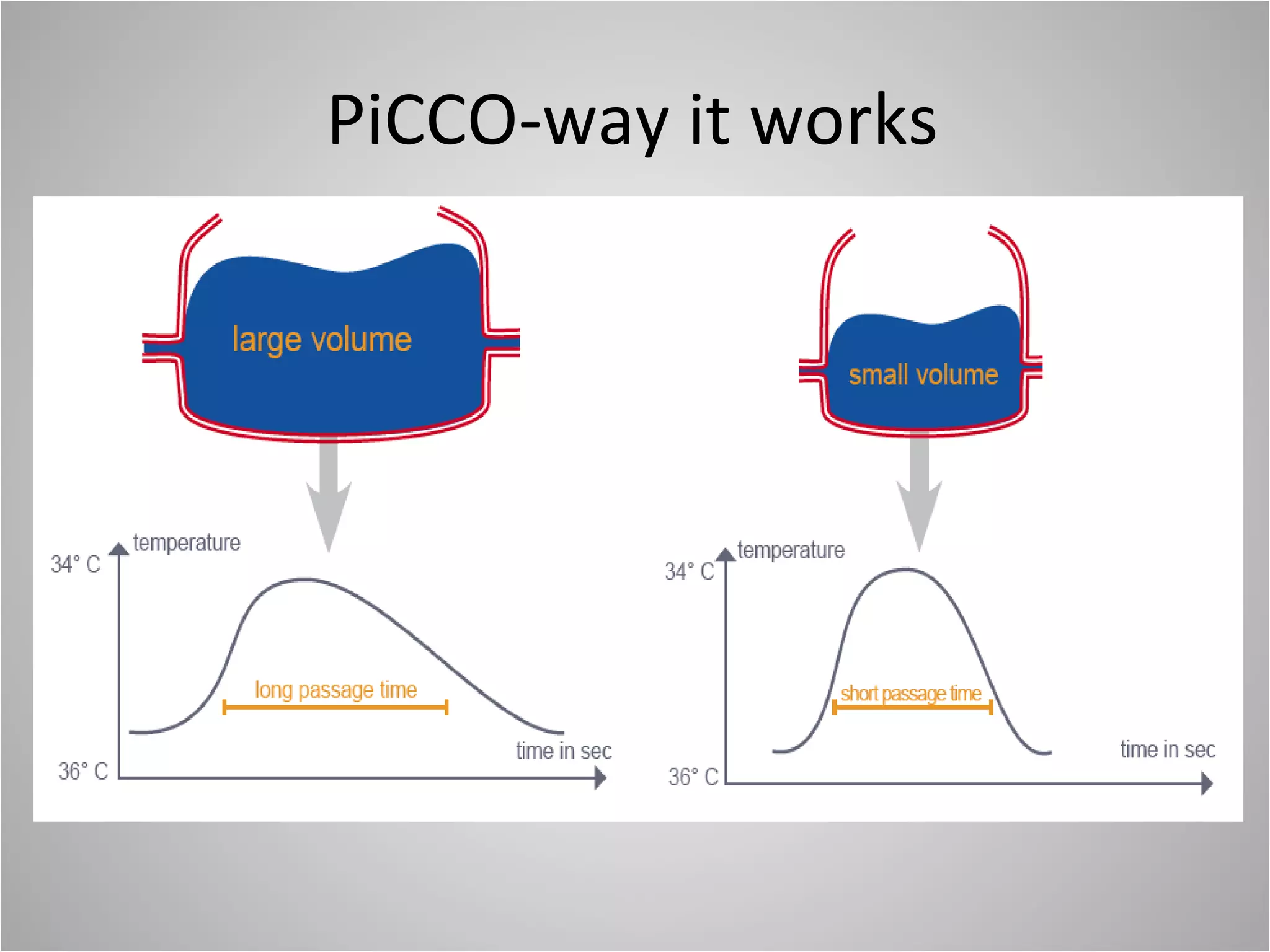
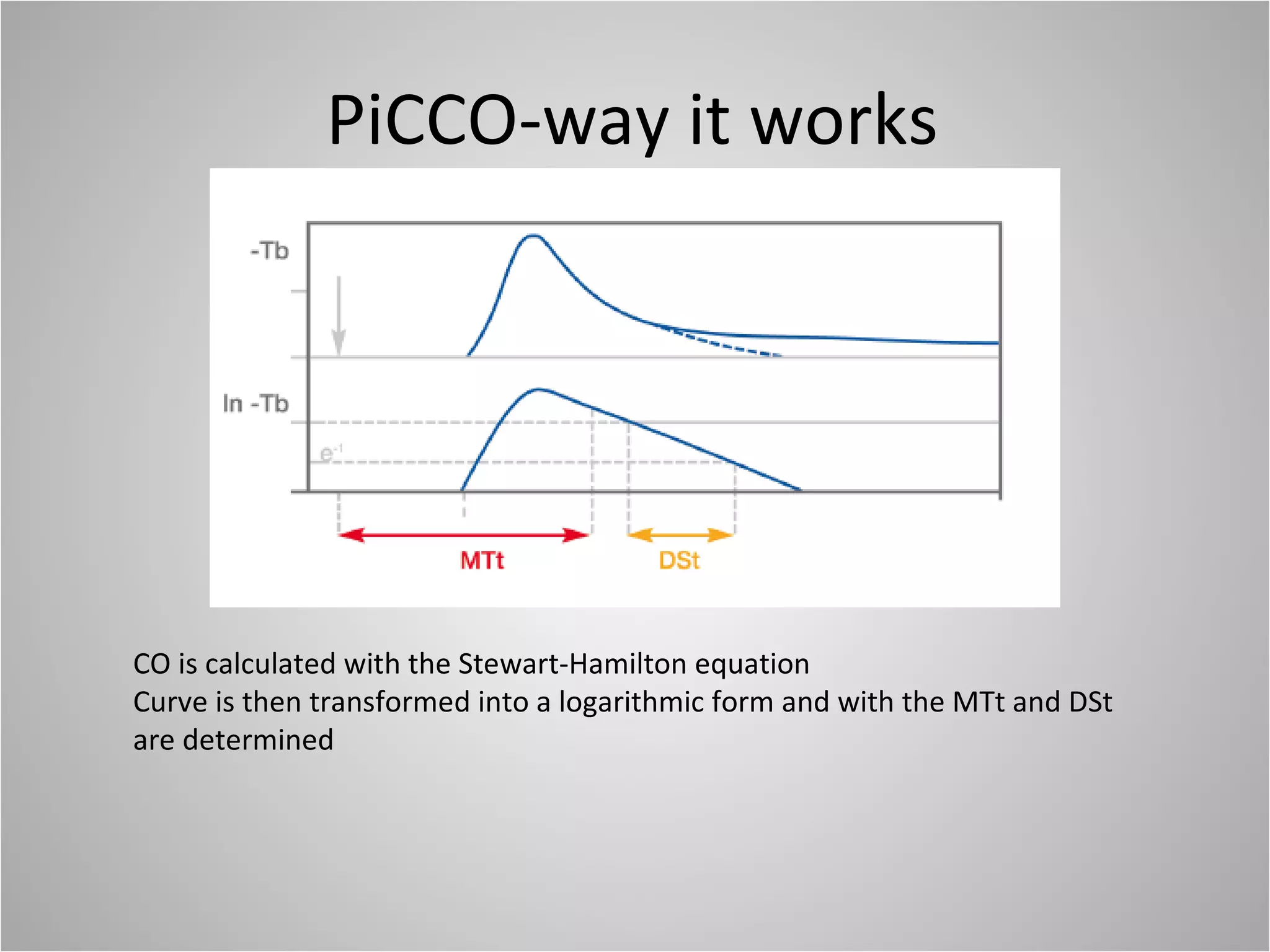
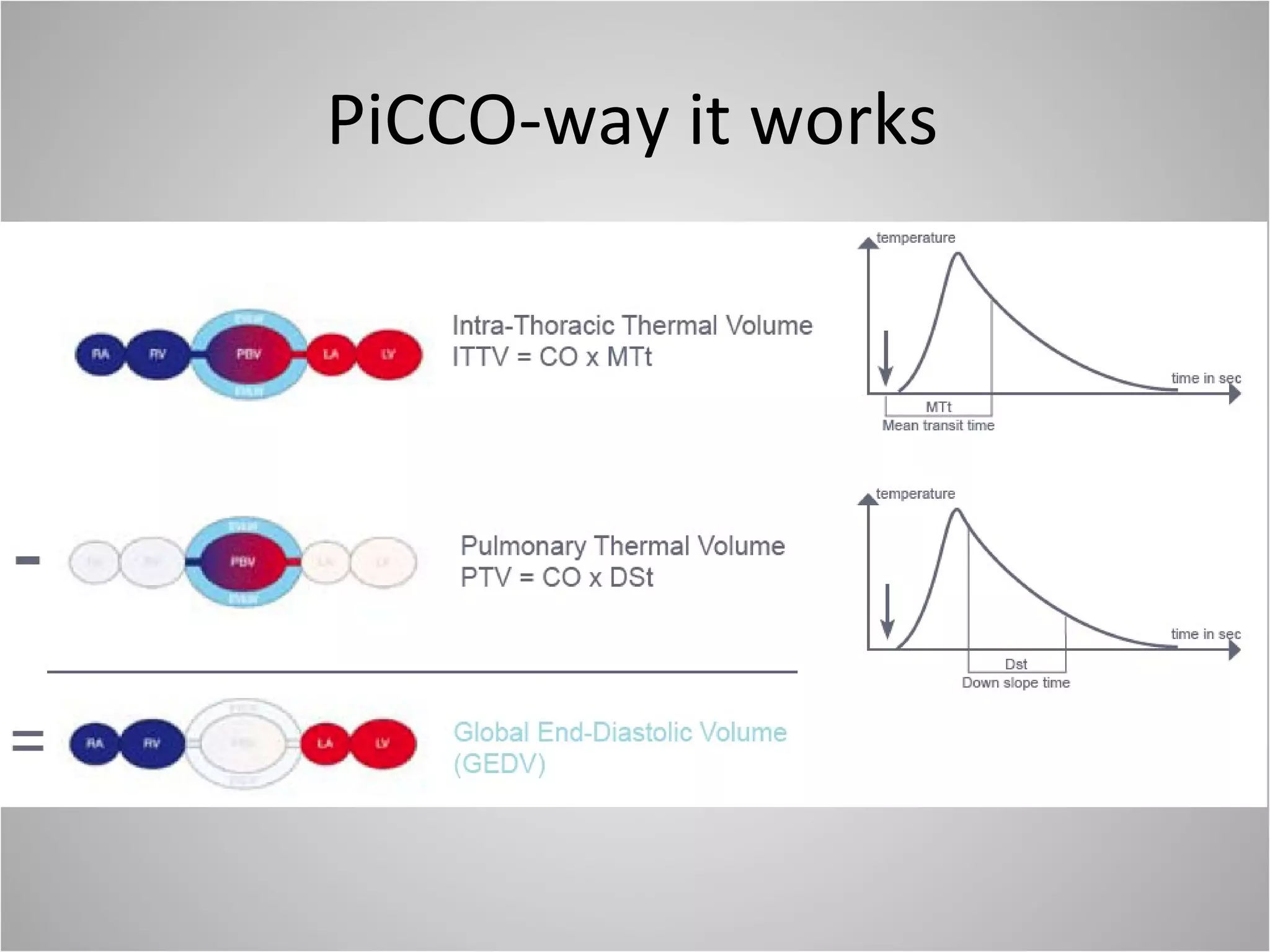
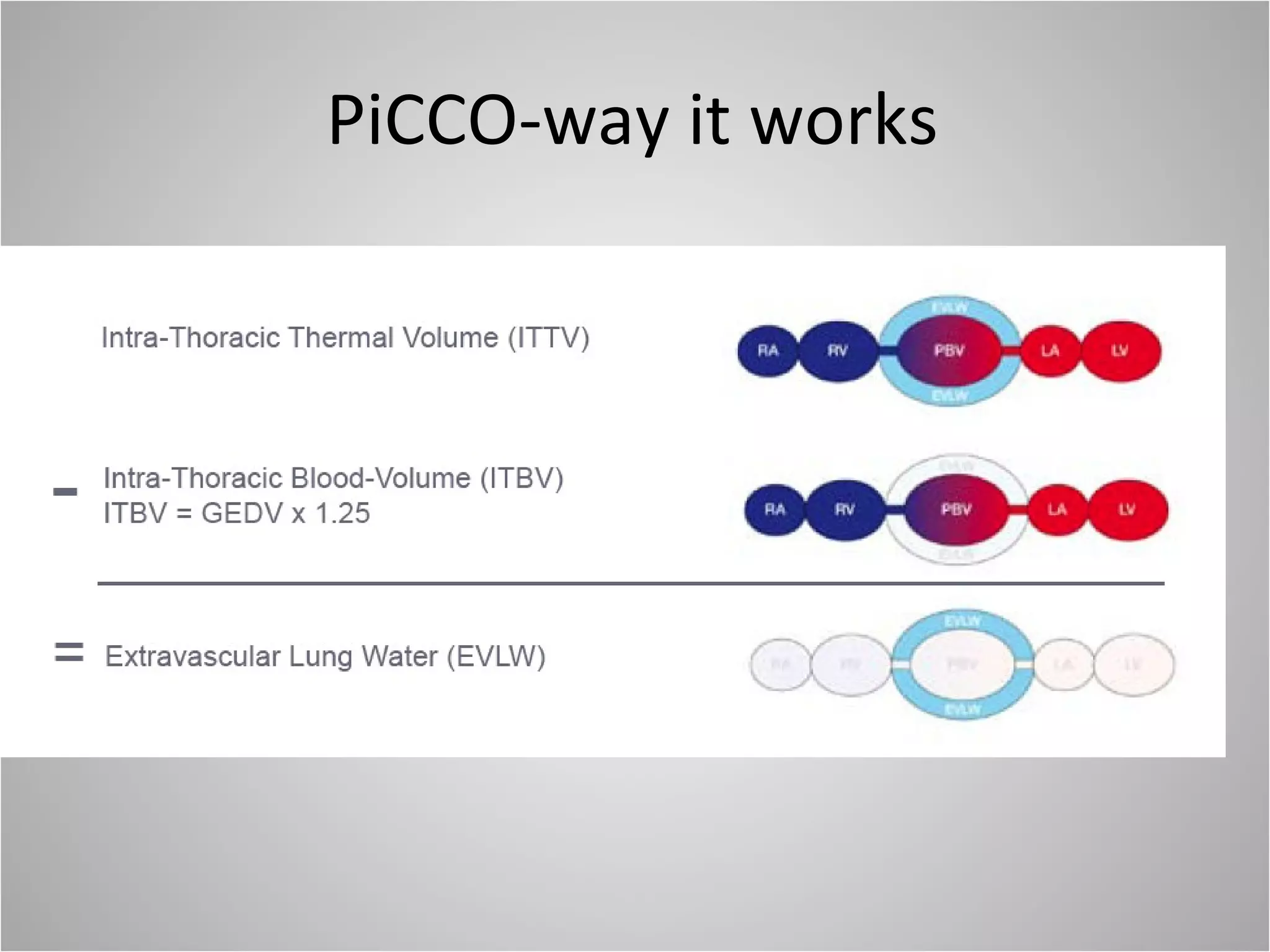
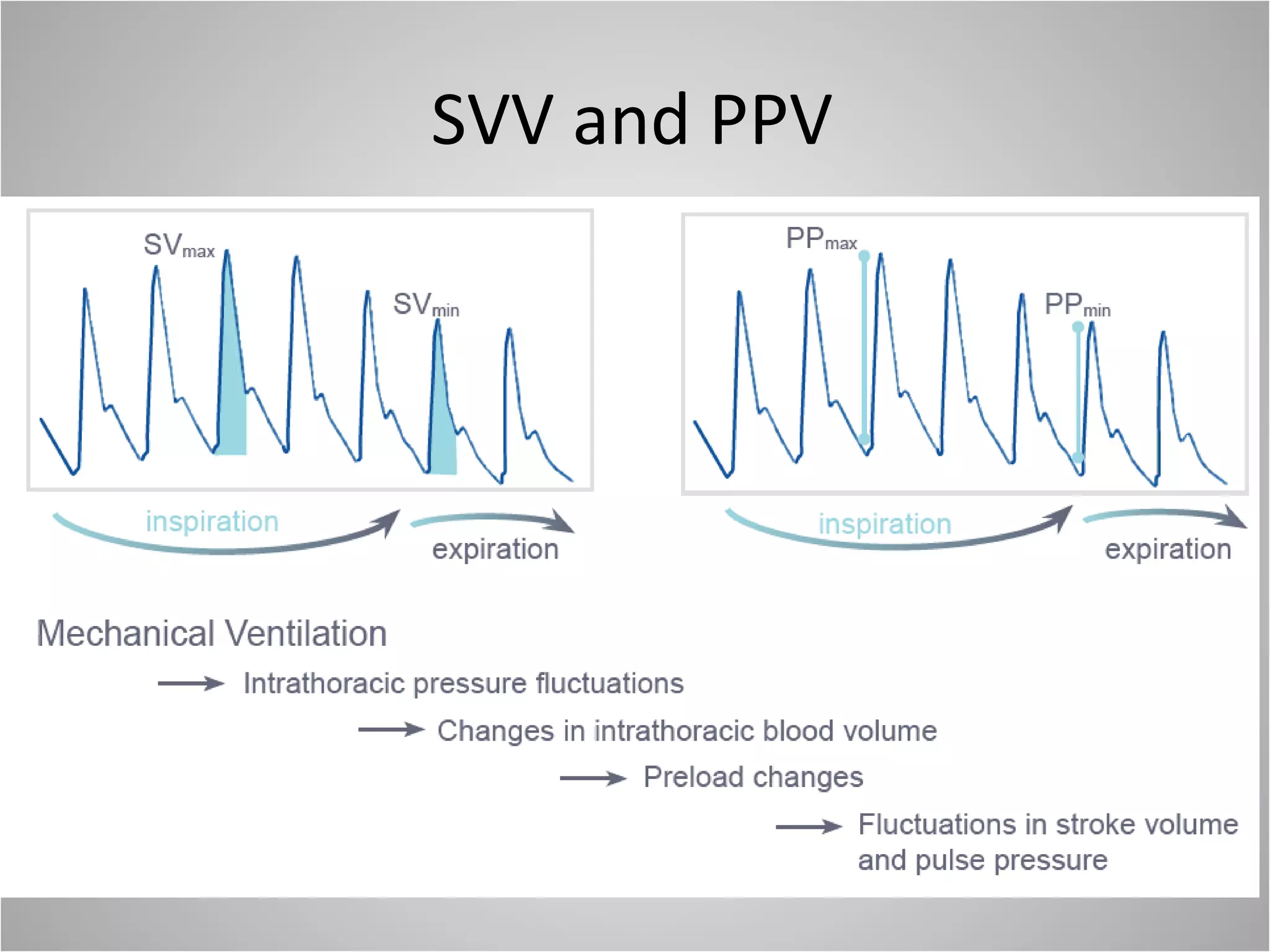
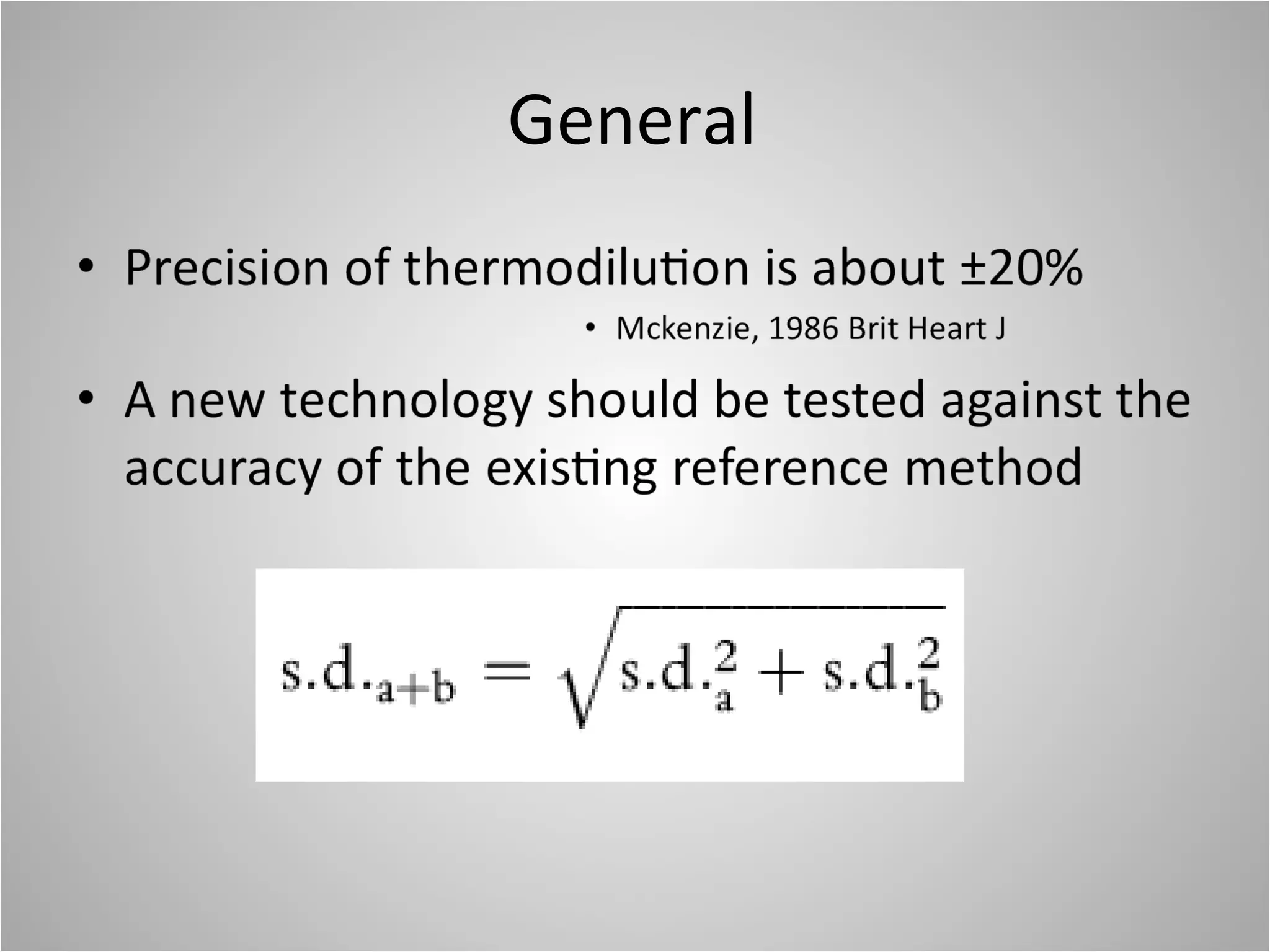
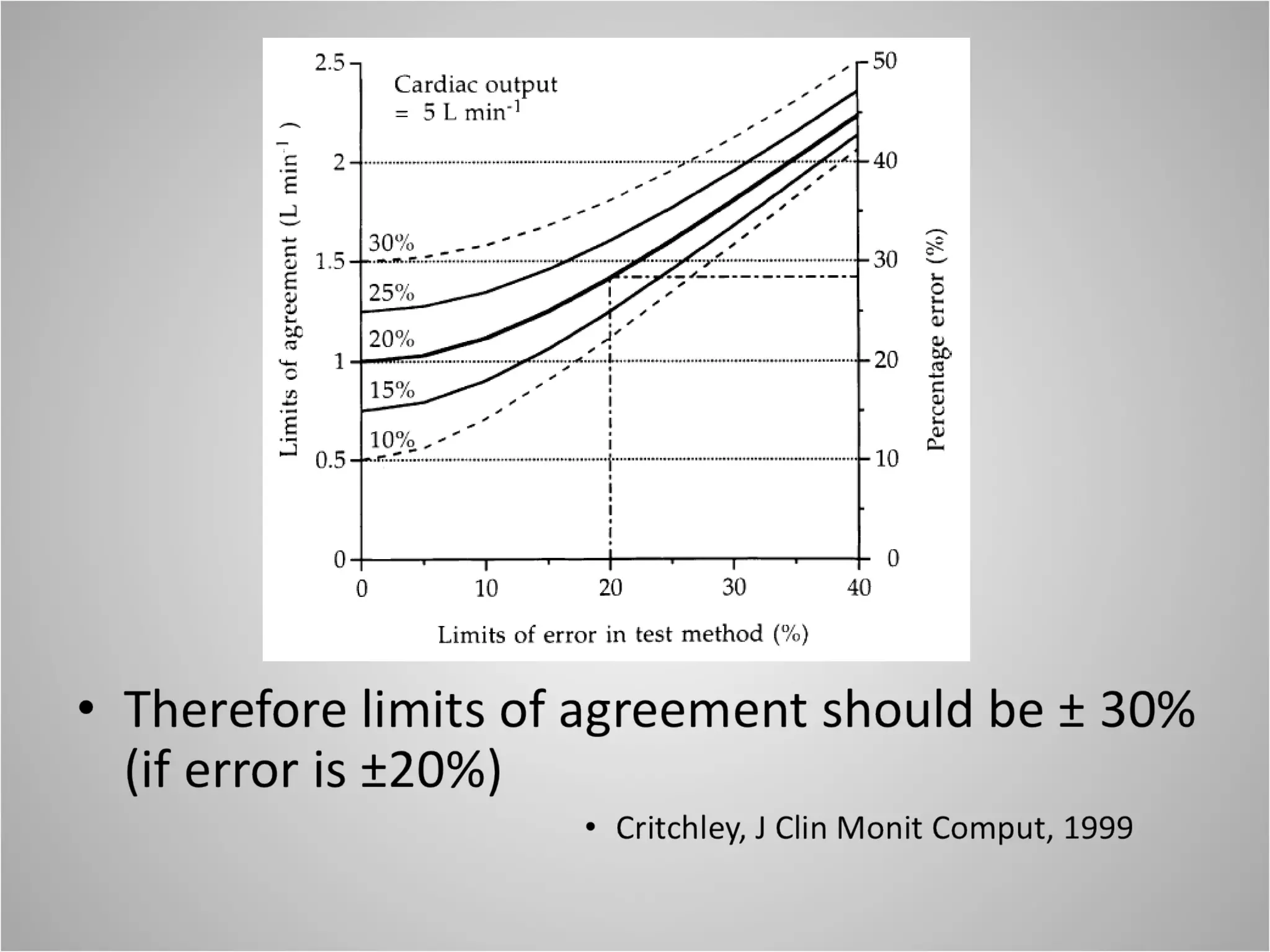
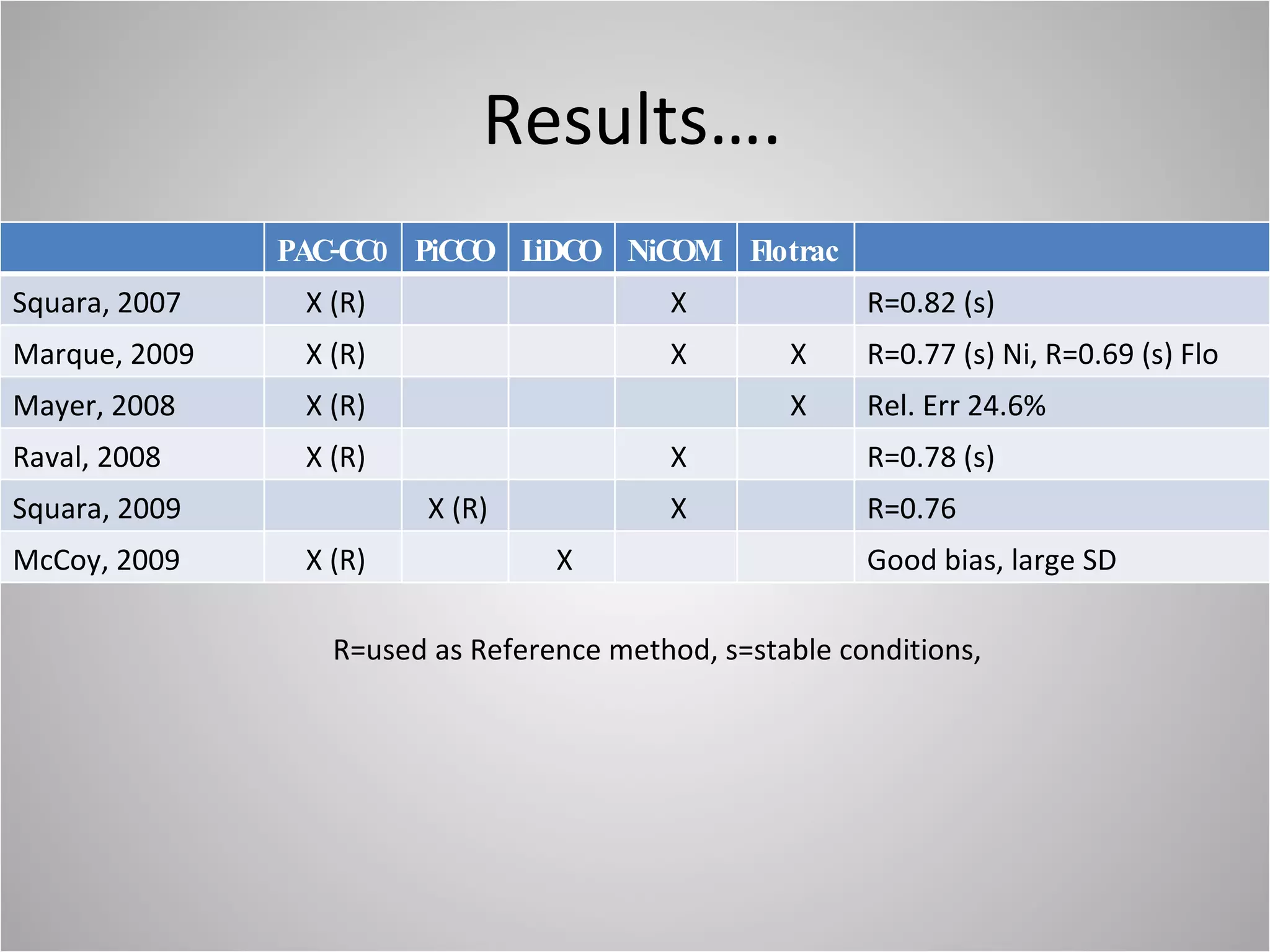
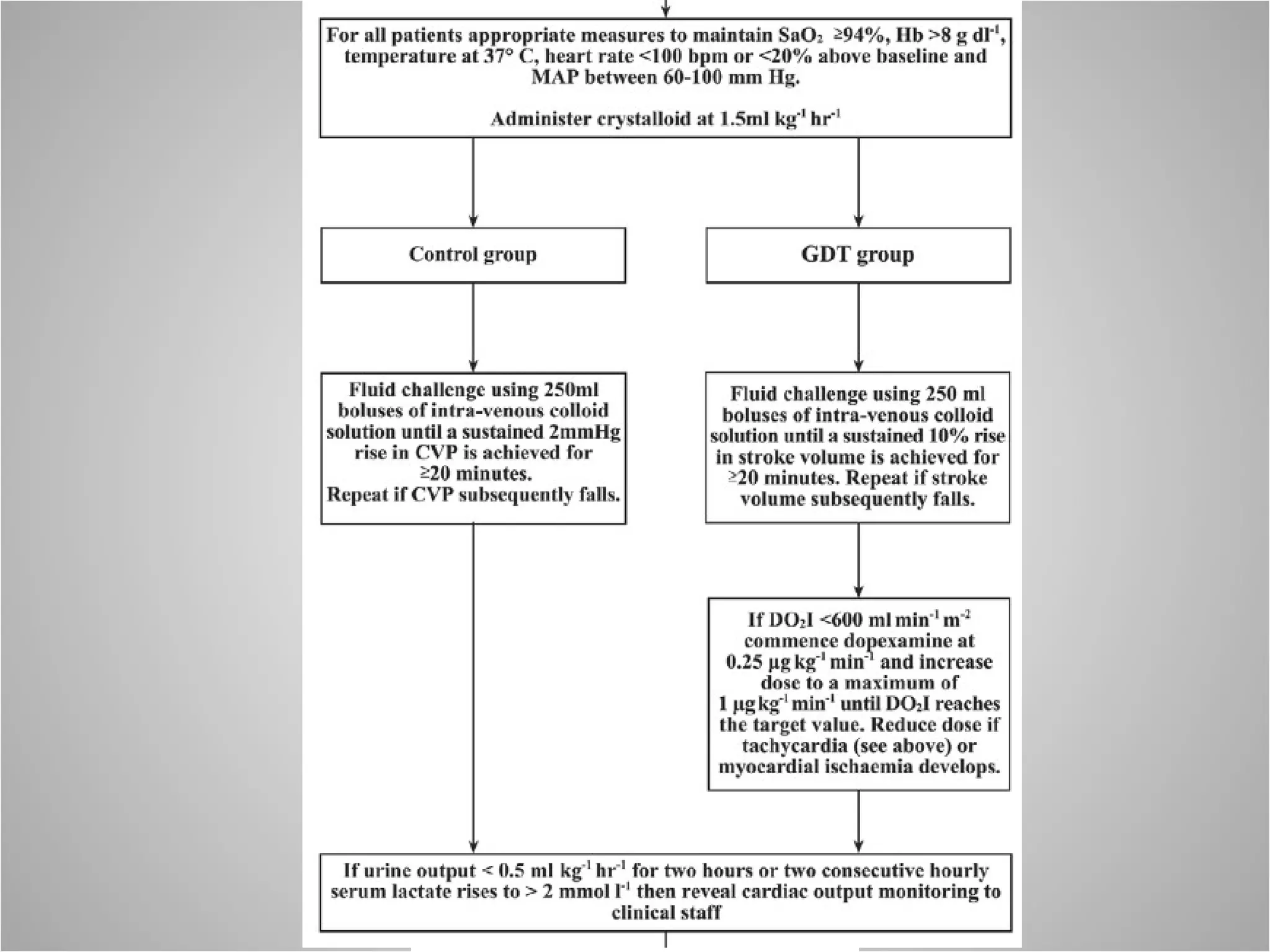
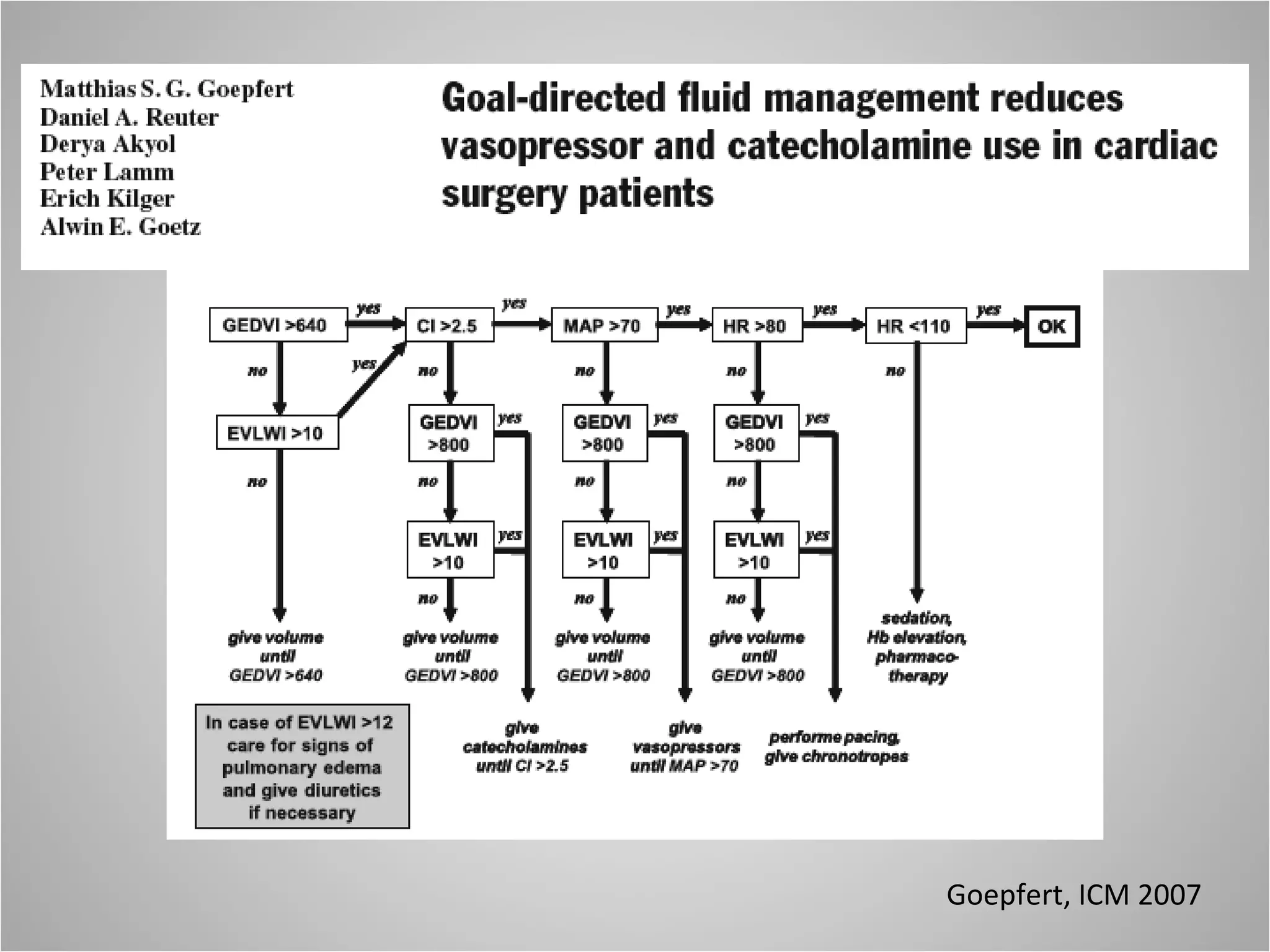
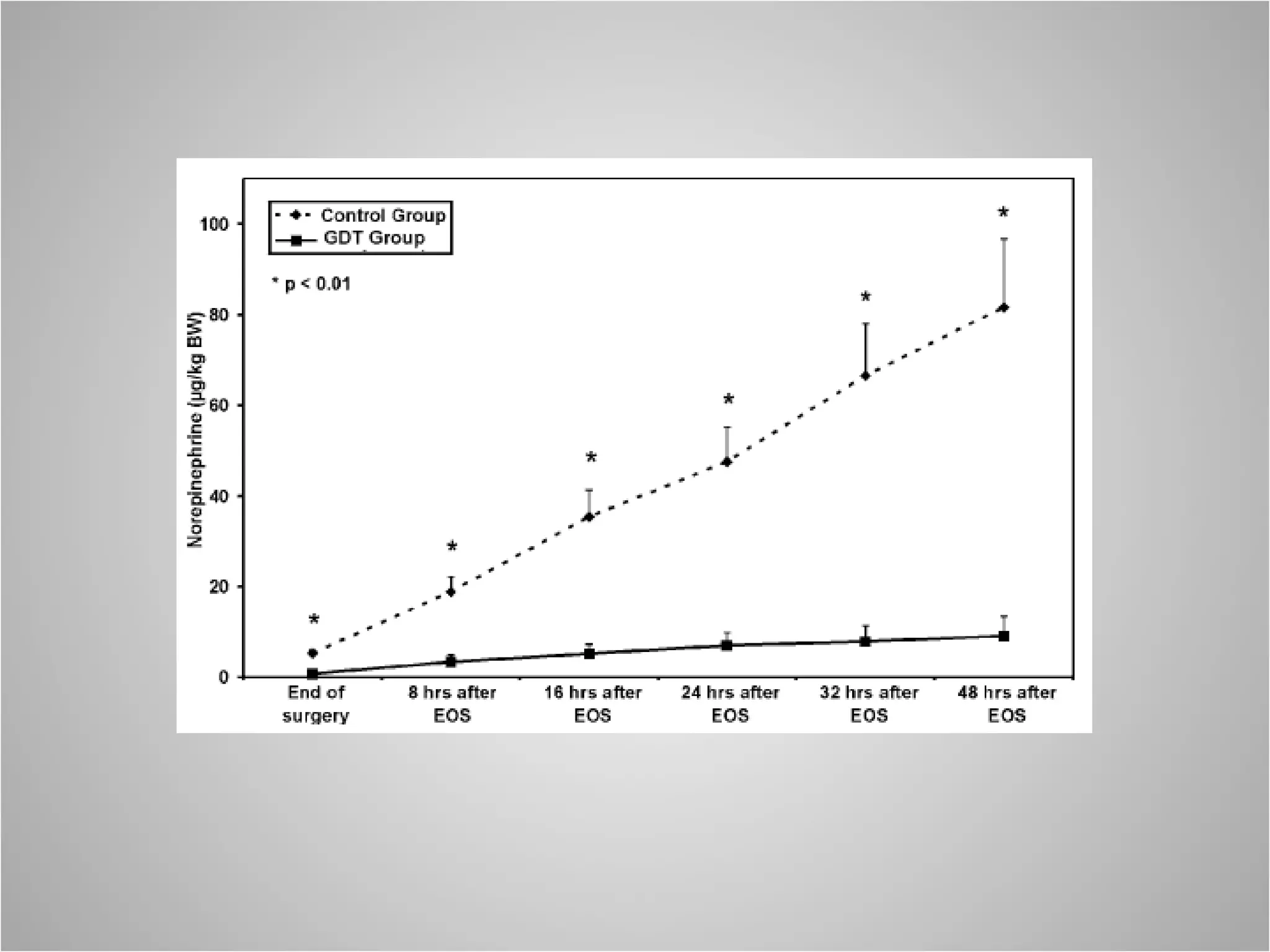
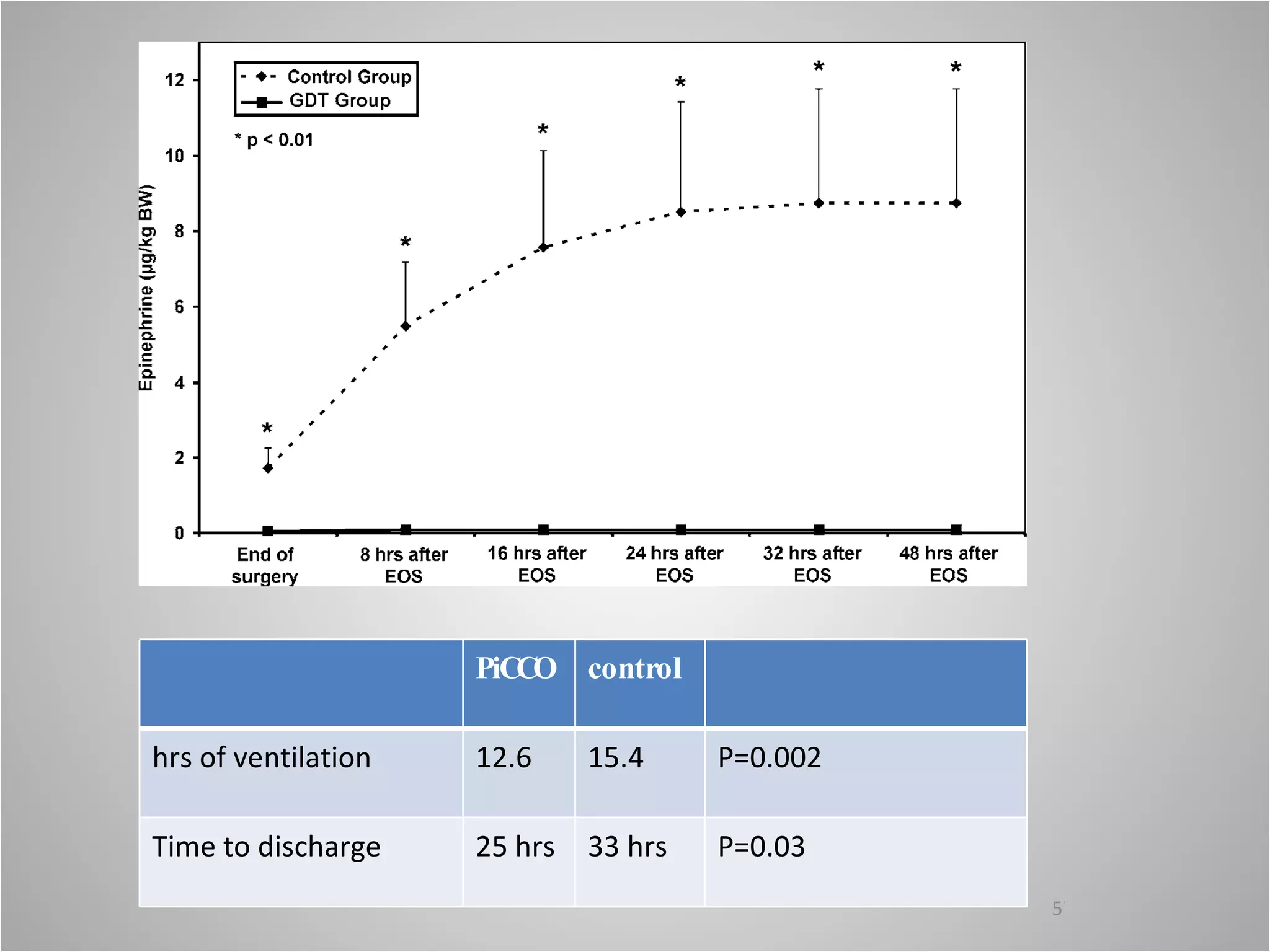
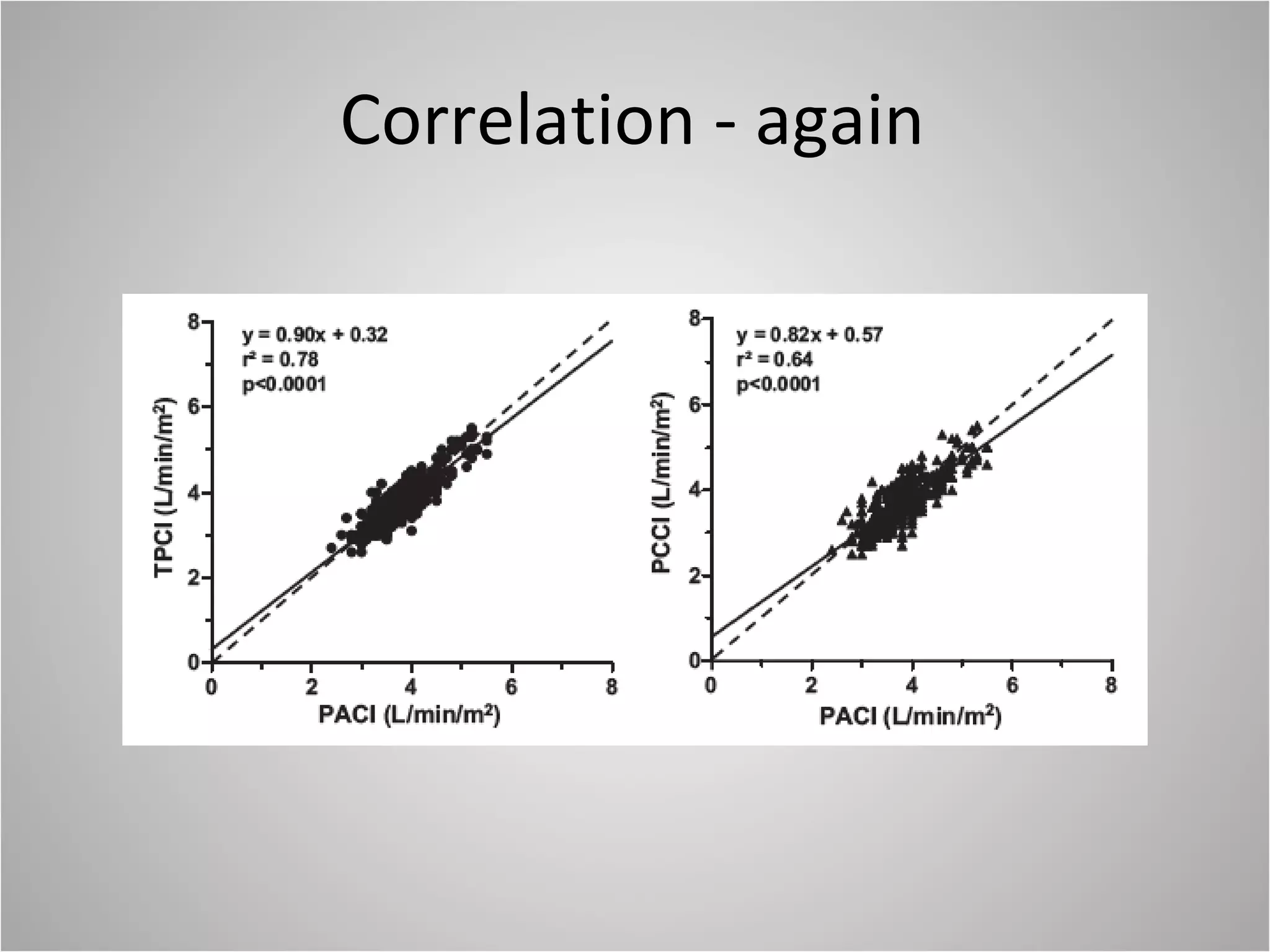
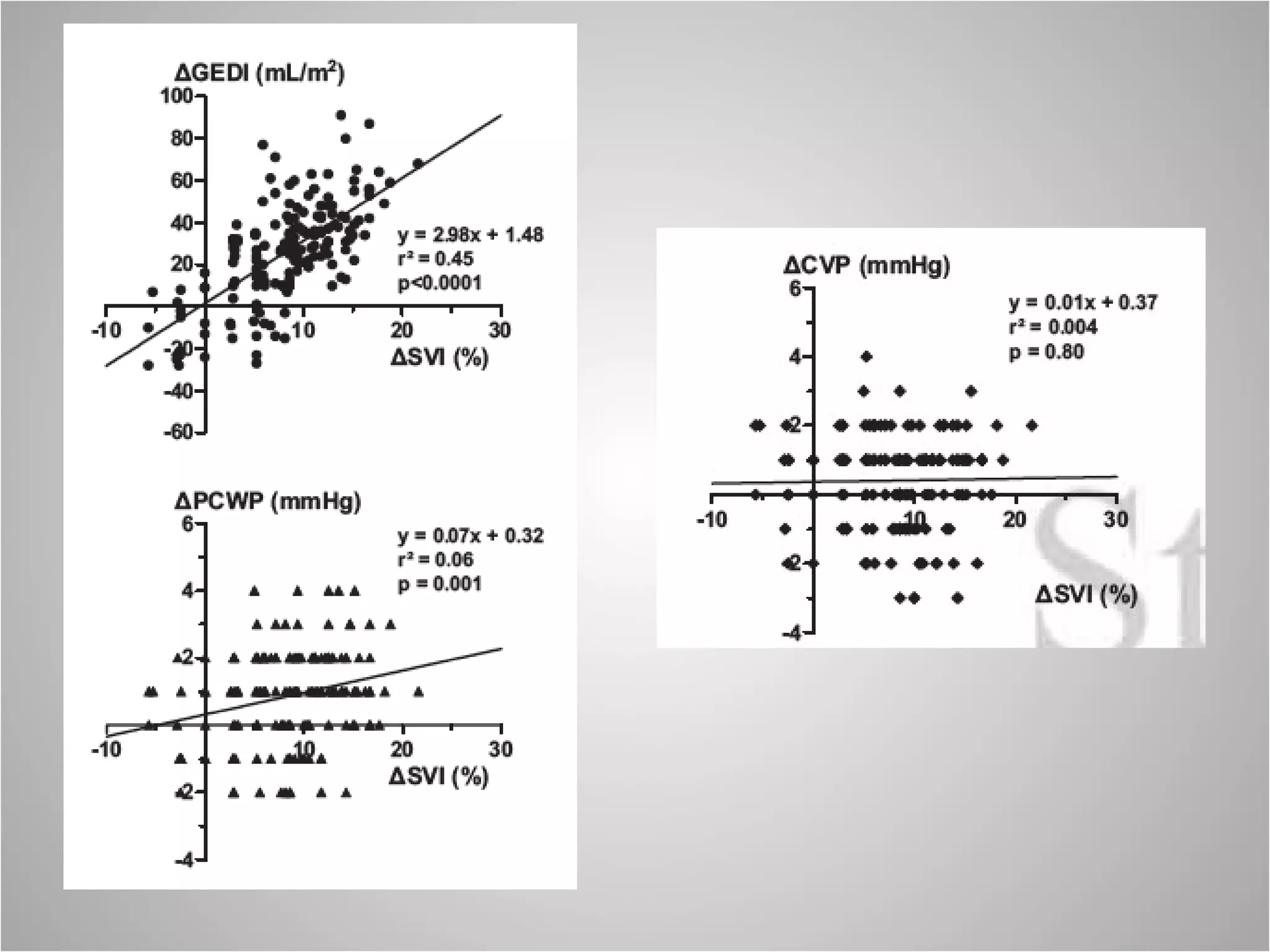
Hemodynamic monitoring involves measuring a patient's circulatory status through various devices. Newer non-invasive devices like bioreactance and pulse contour analysis aim to continuously monitor cardiac output without needing a pulmonary artery catheter. Clinical trials show mixed results on whether advanced hemodynamic monitoring improves outcomes, but some evidence suggests it can reduce complications, length of hospital stay, and ventilation time in high-risk patients.