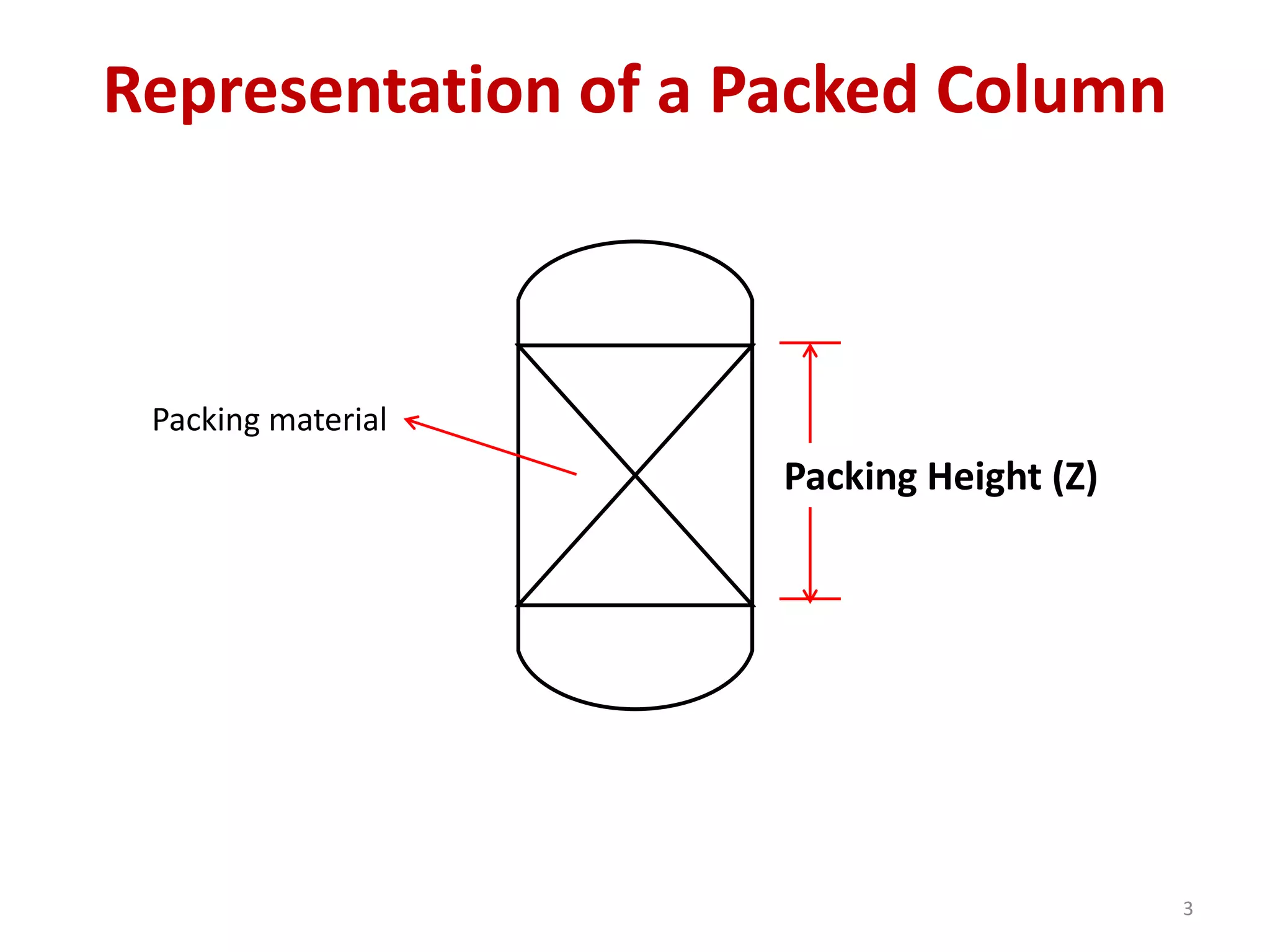
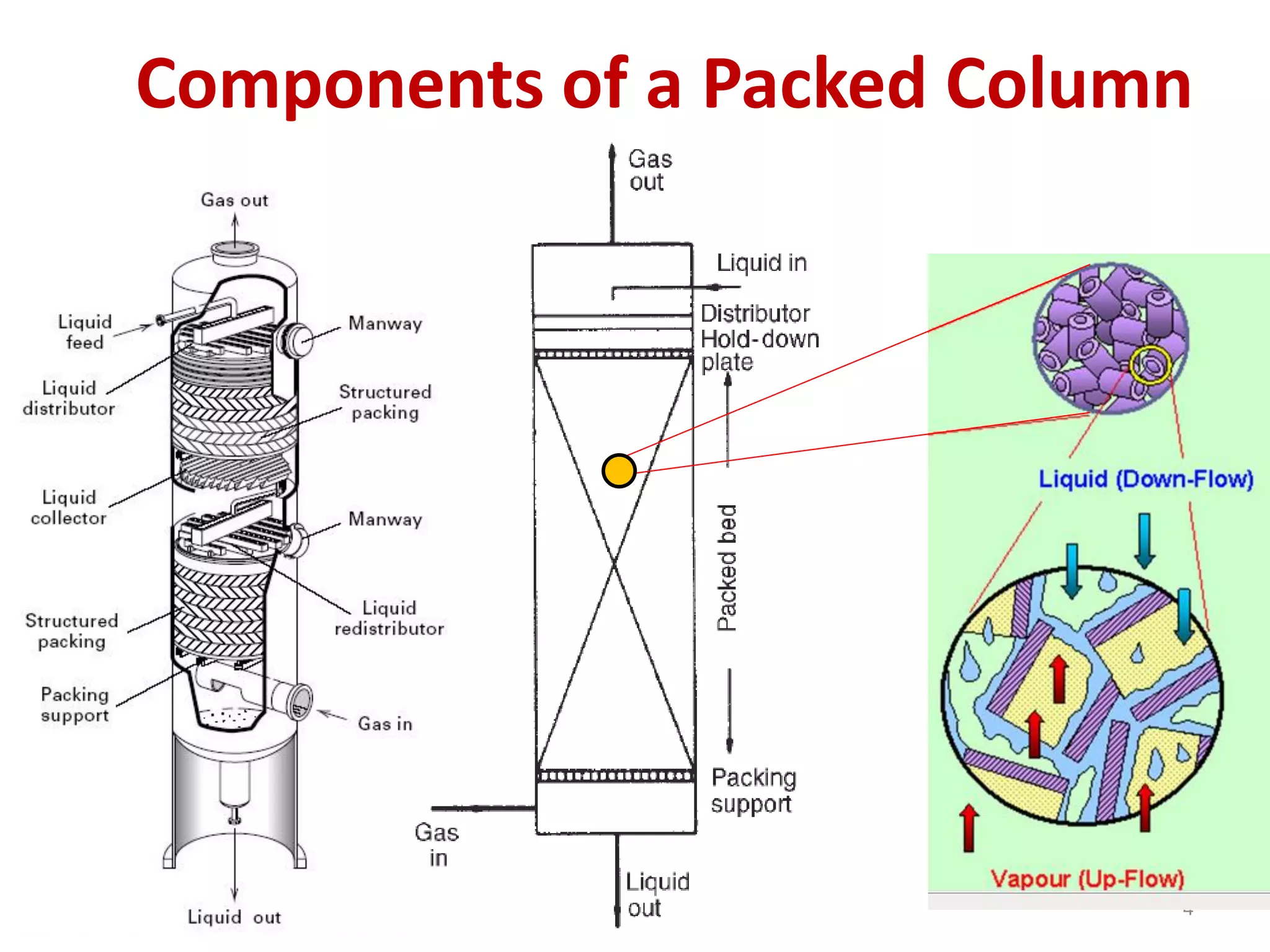
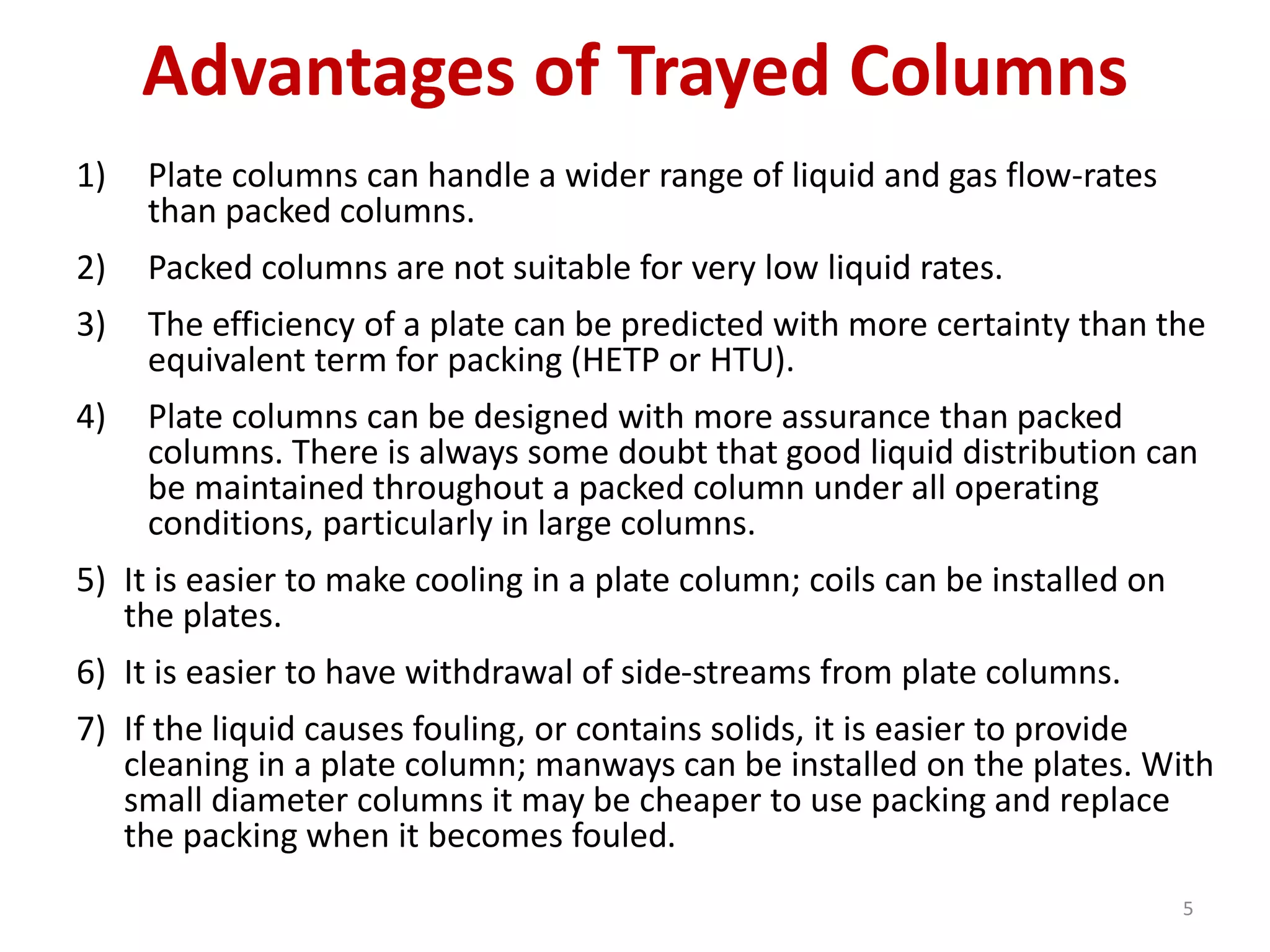
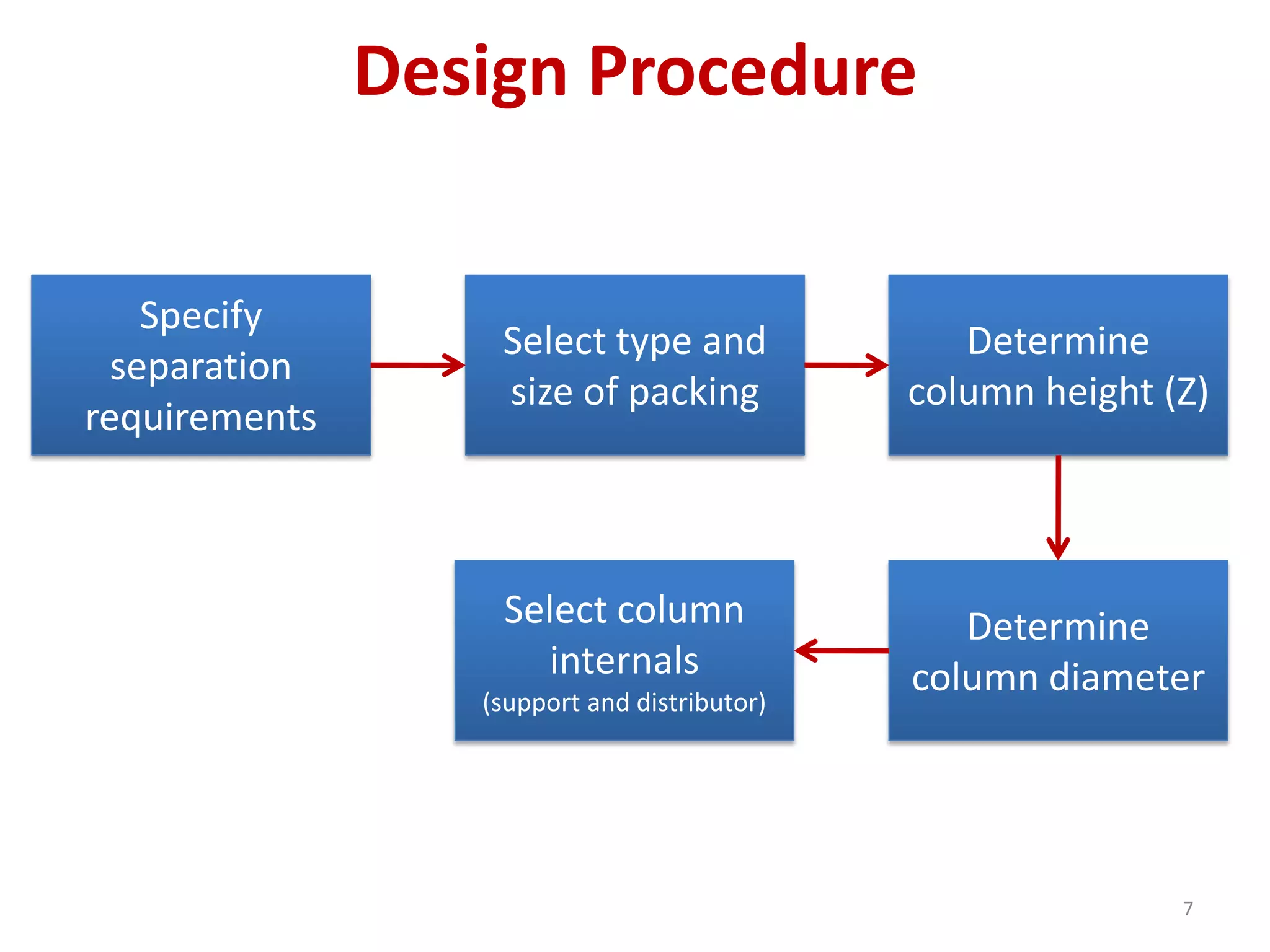
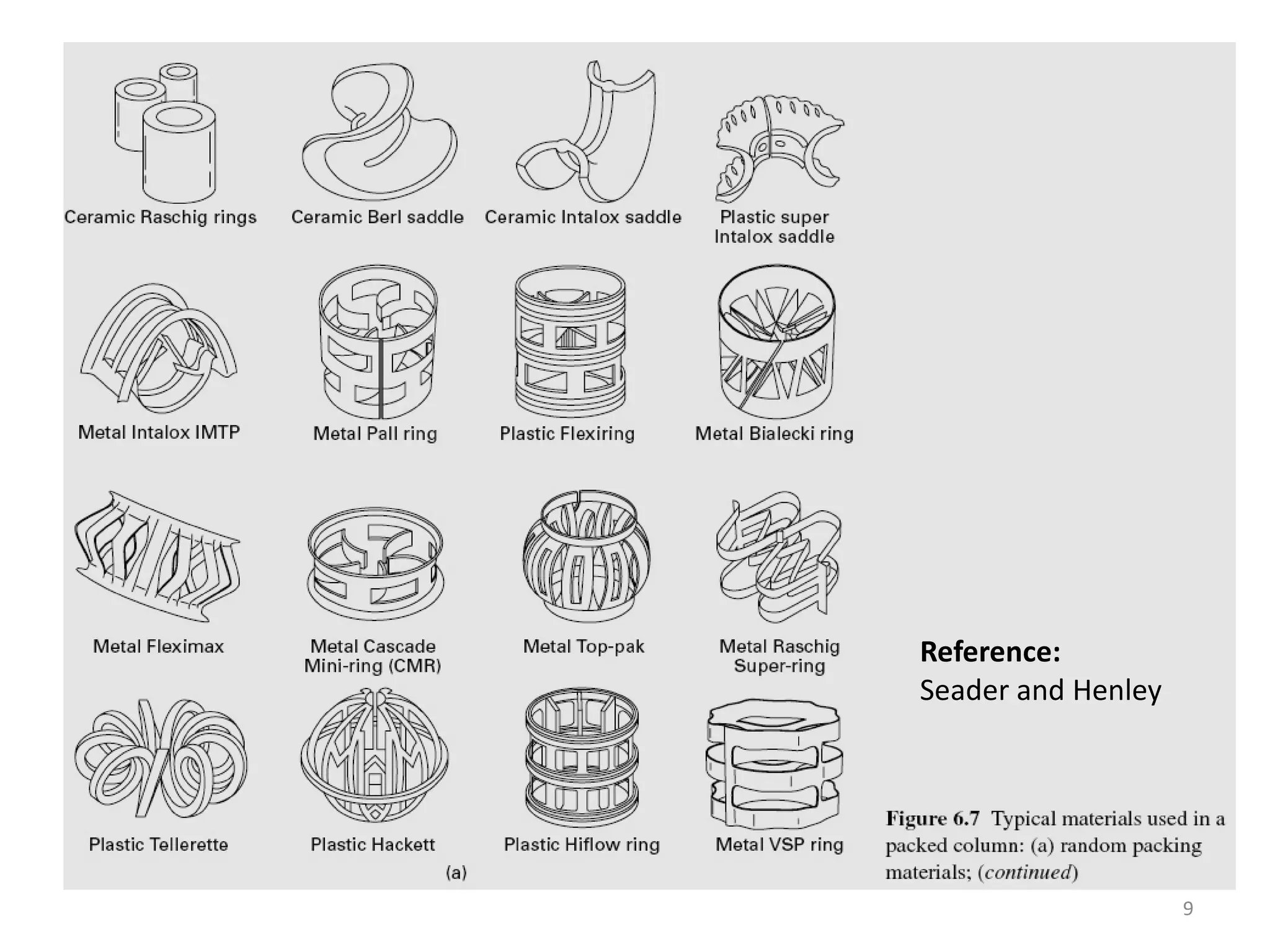
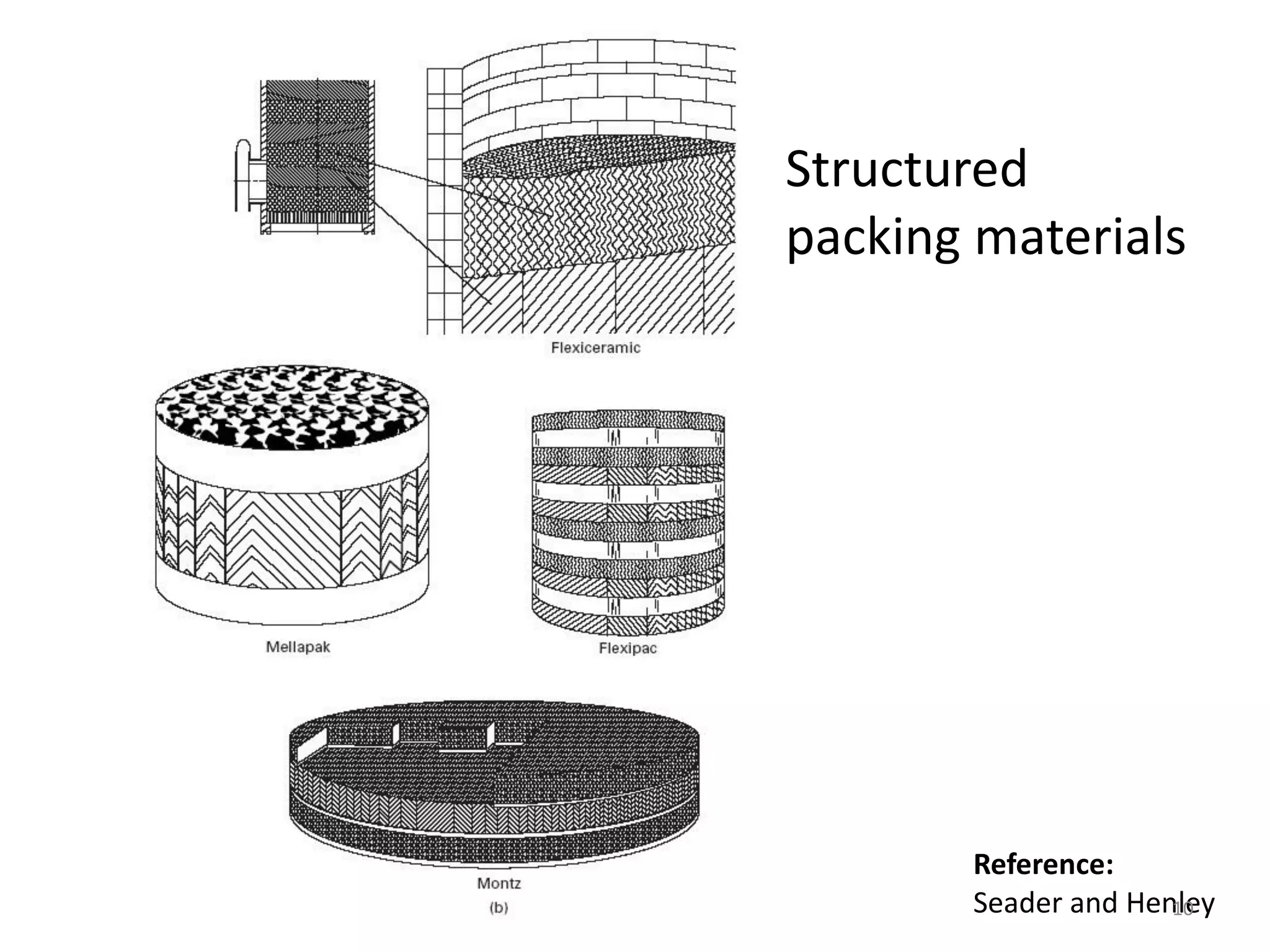
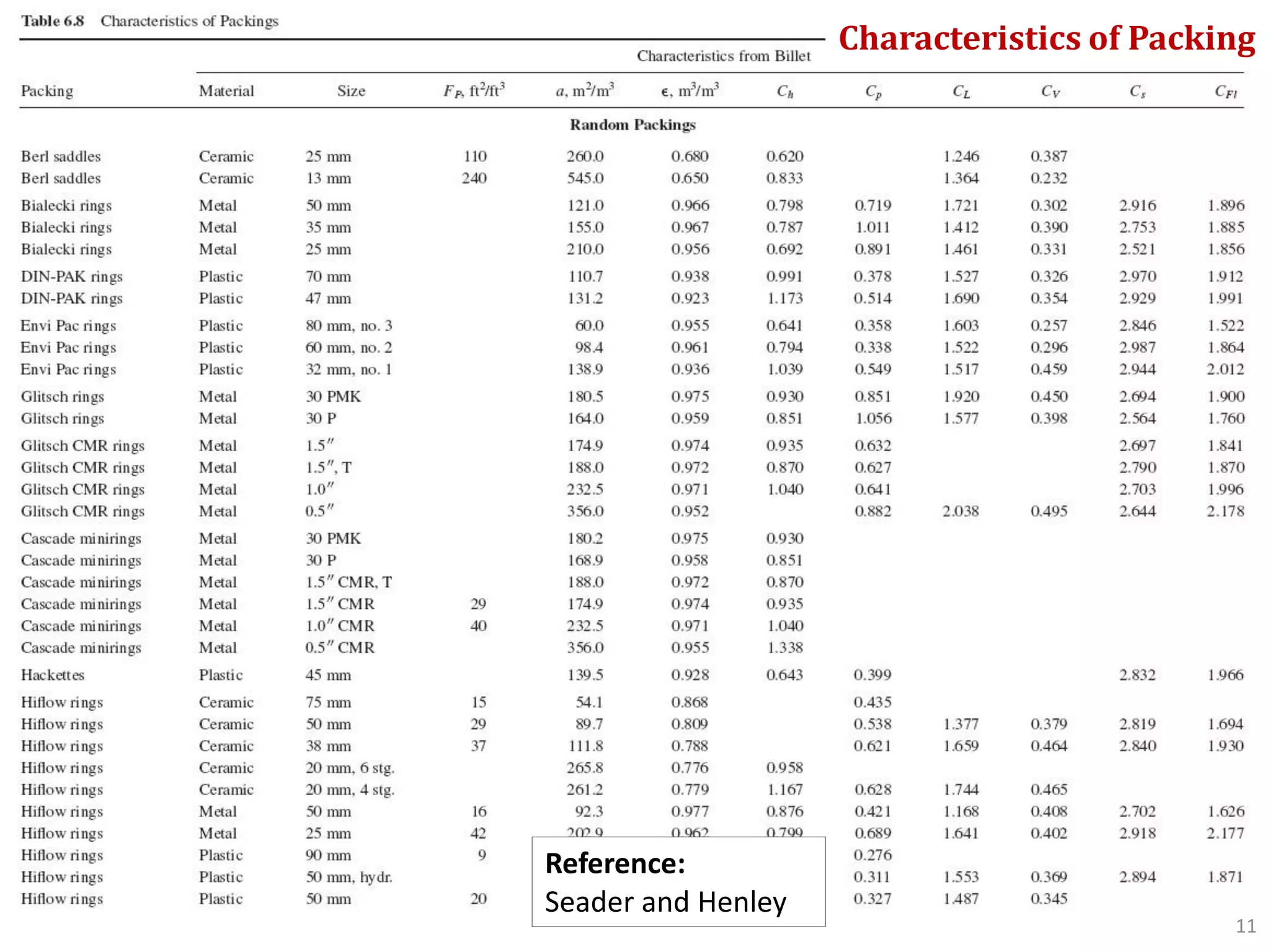
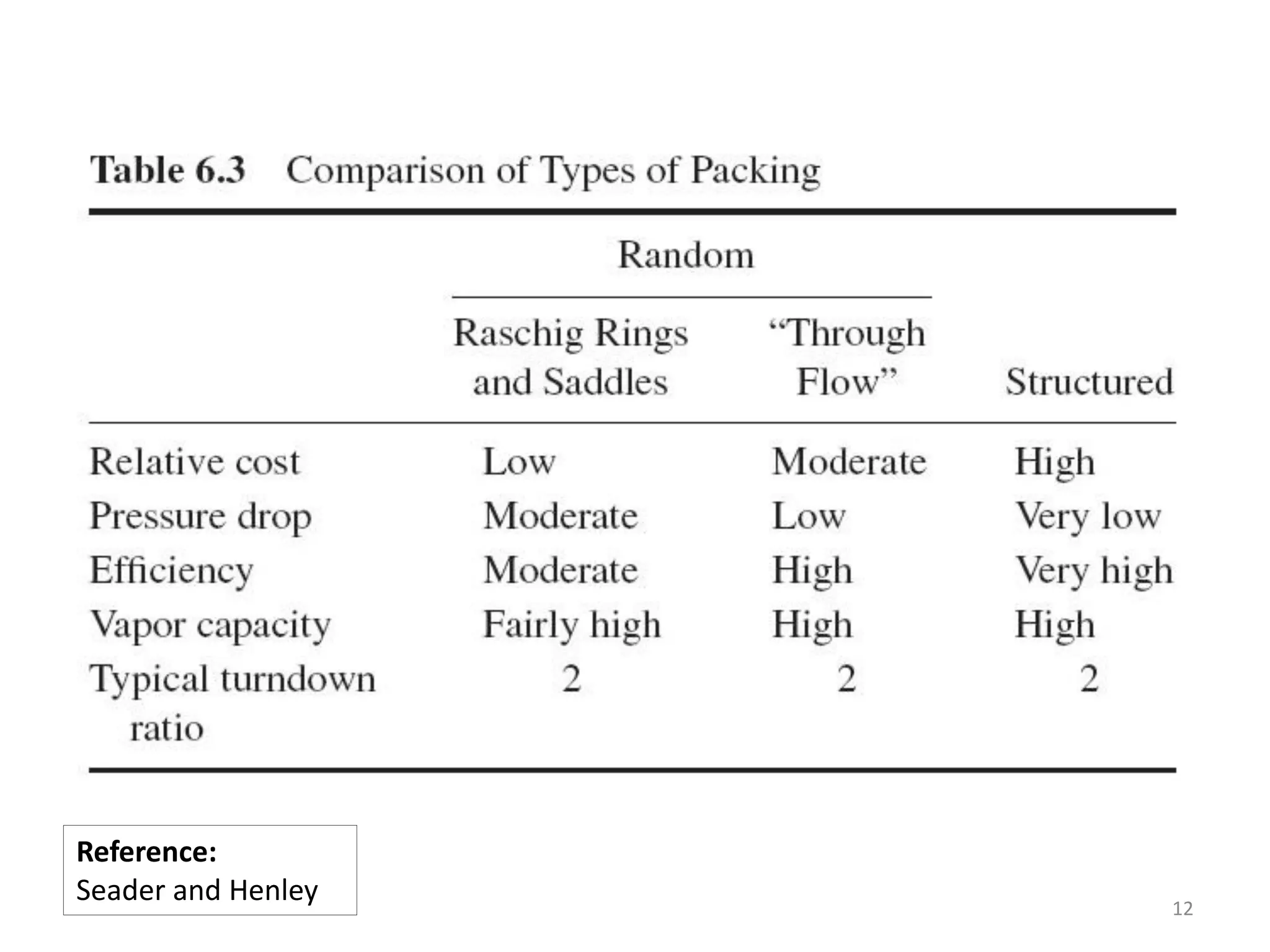
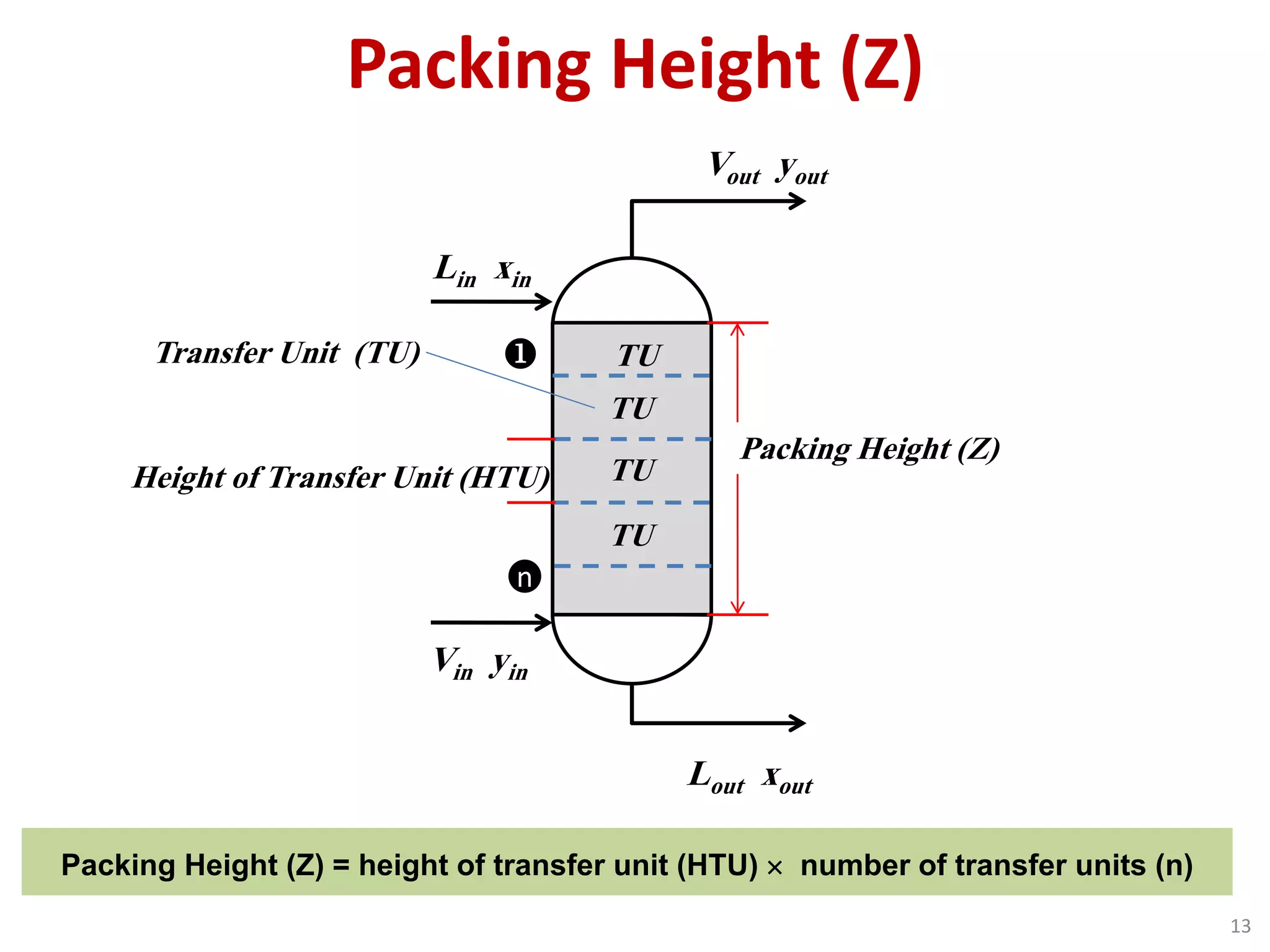
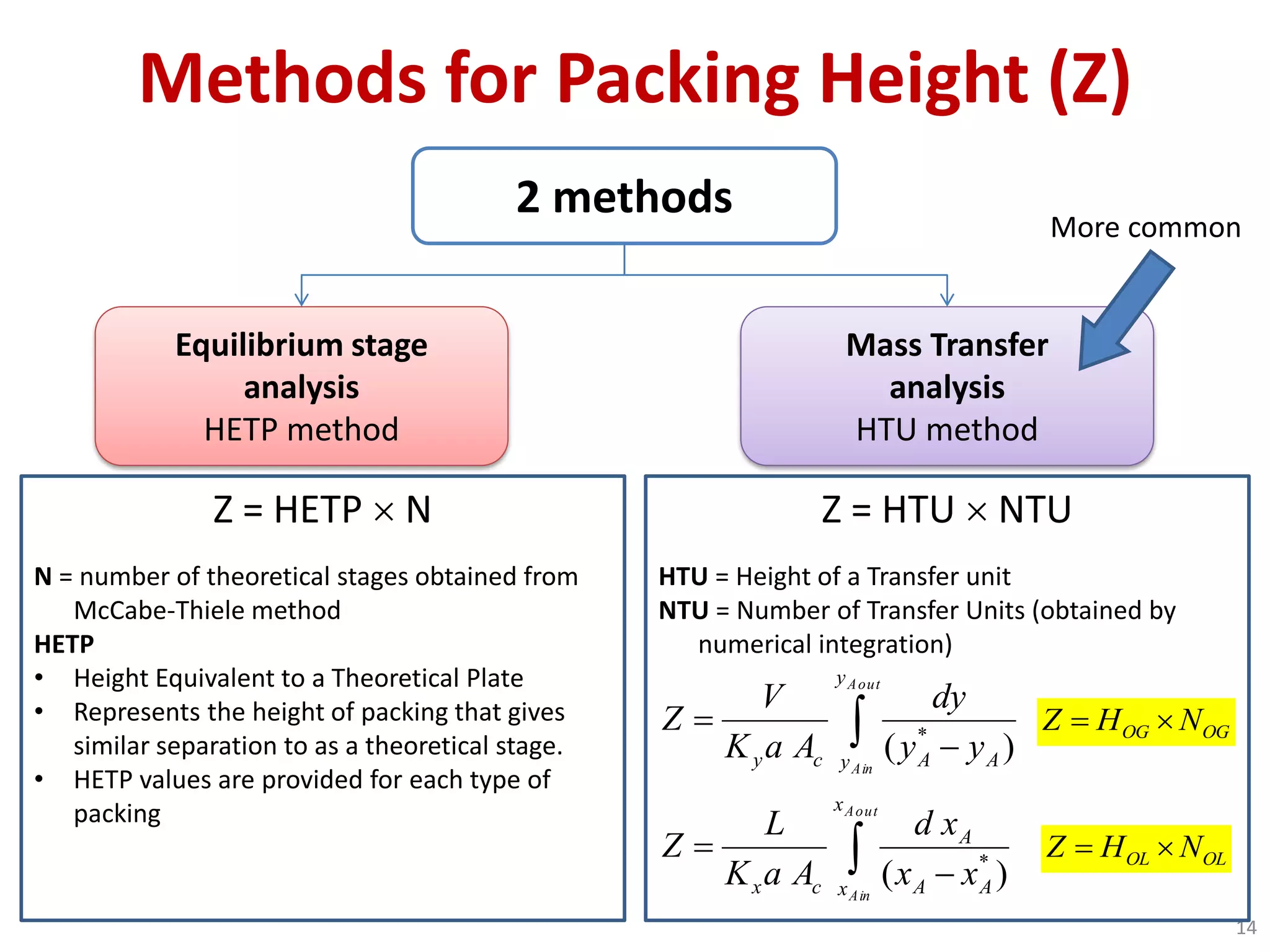
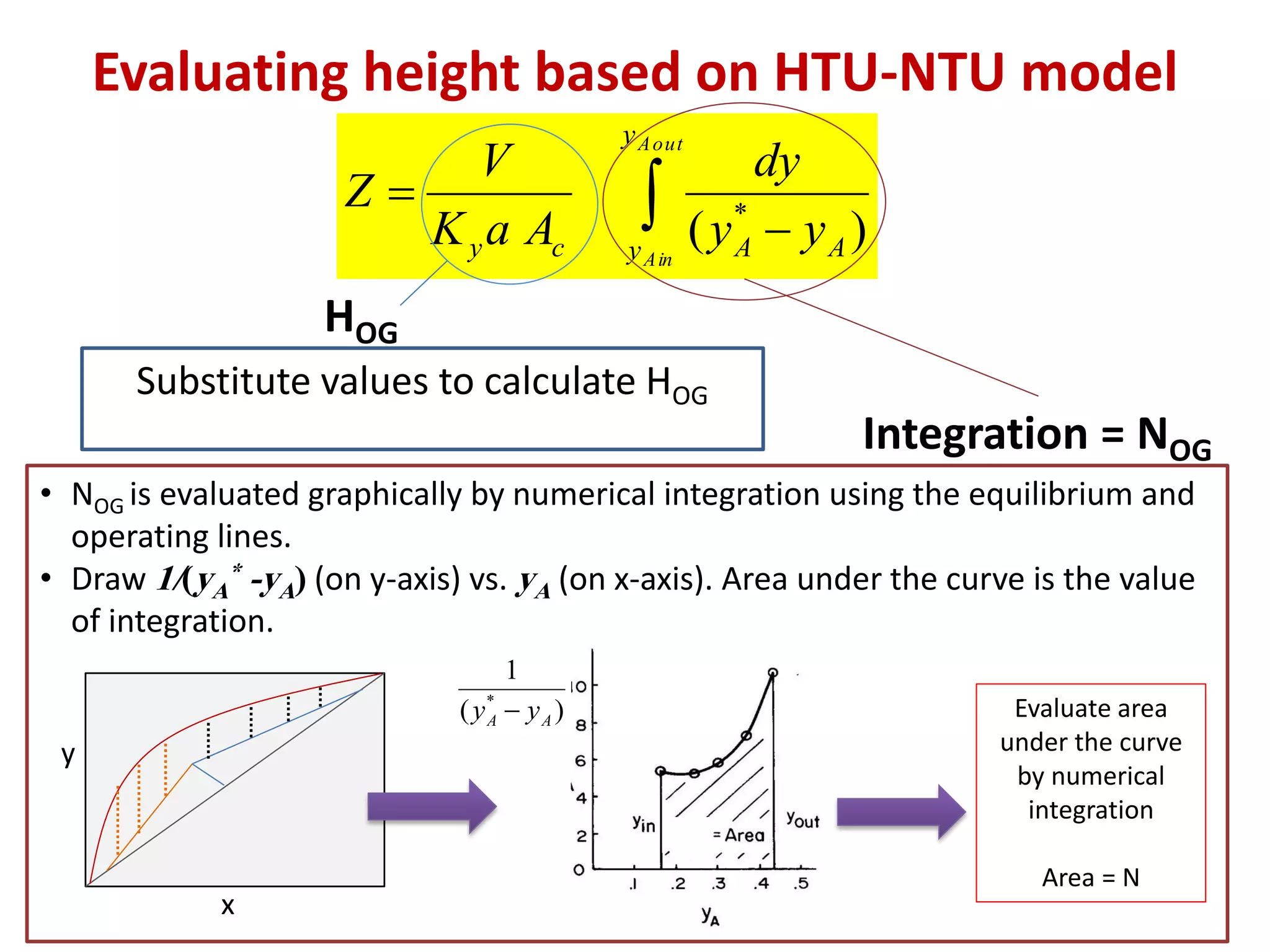
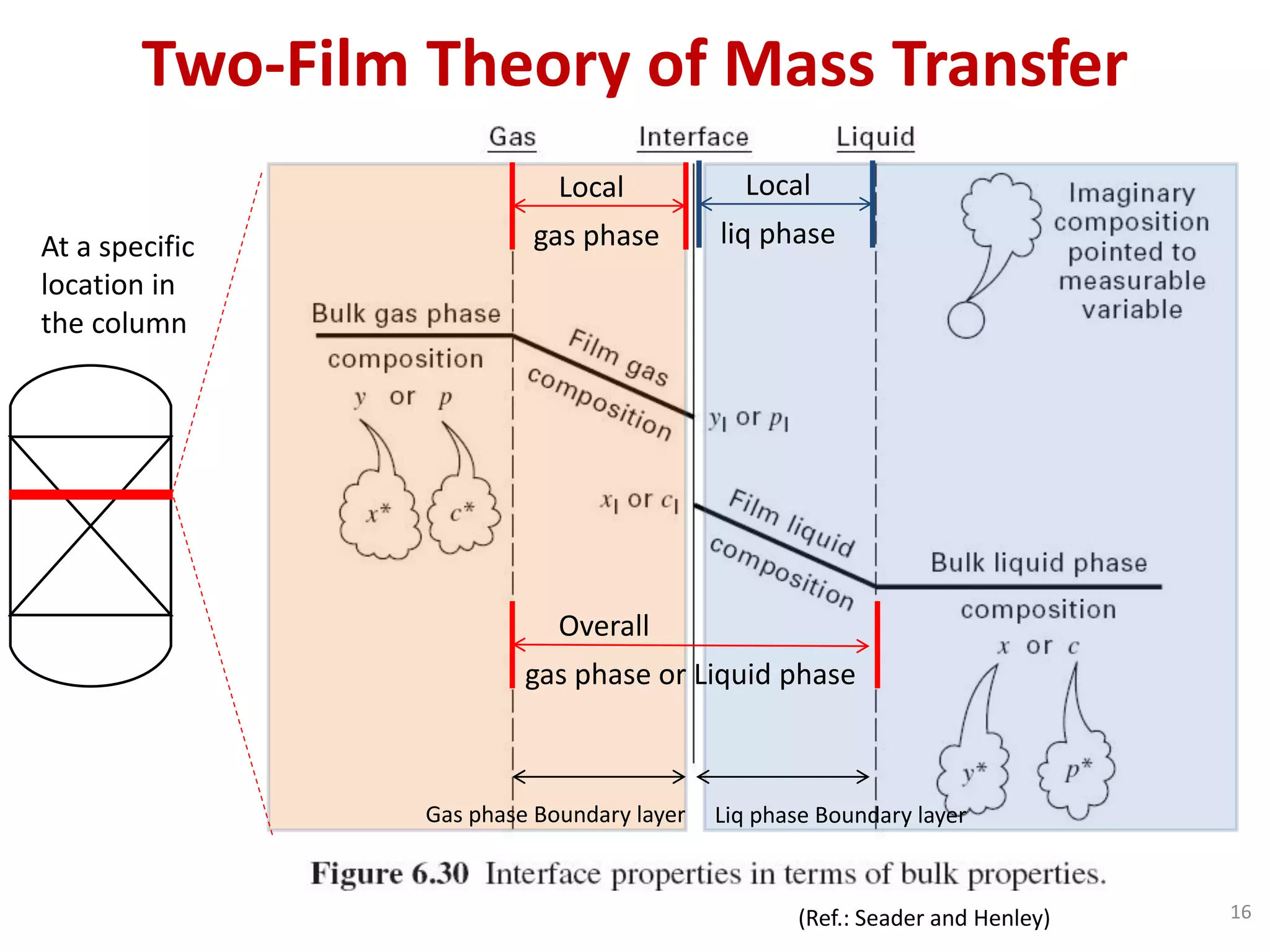
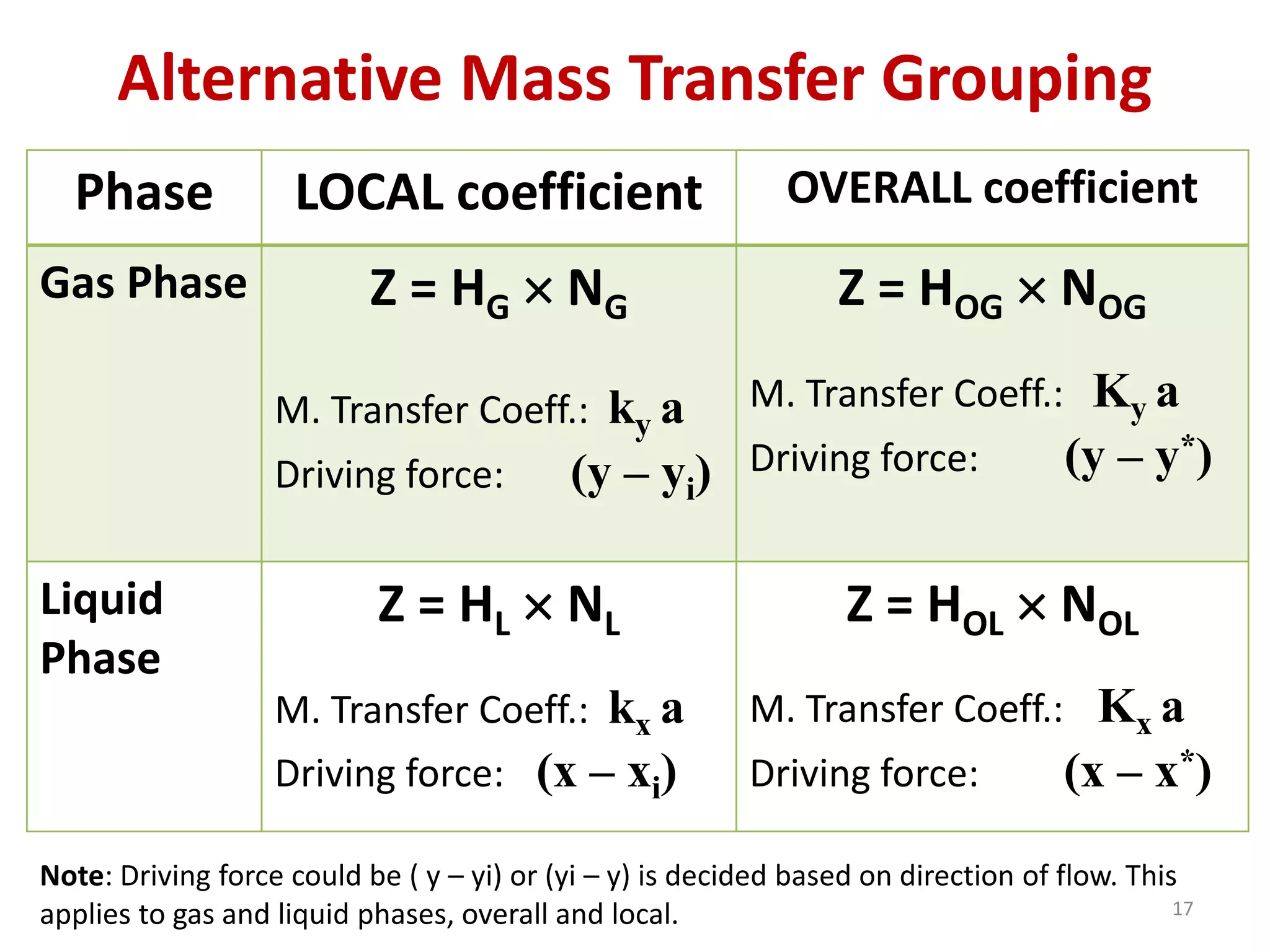
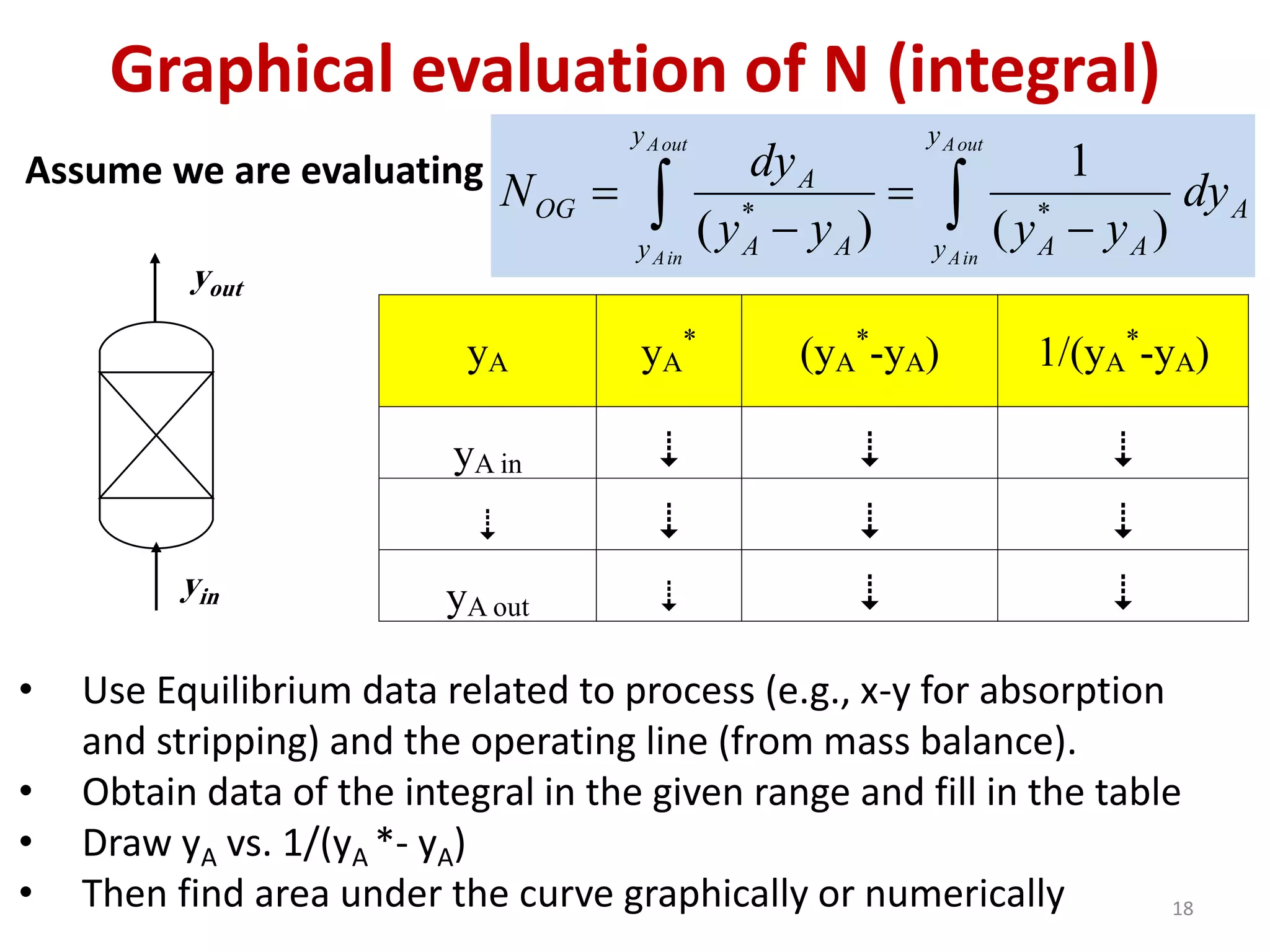
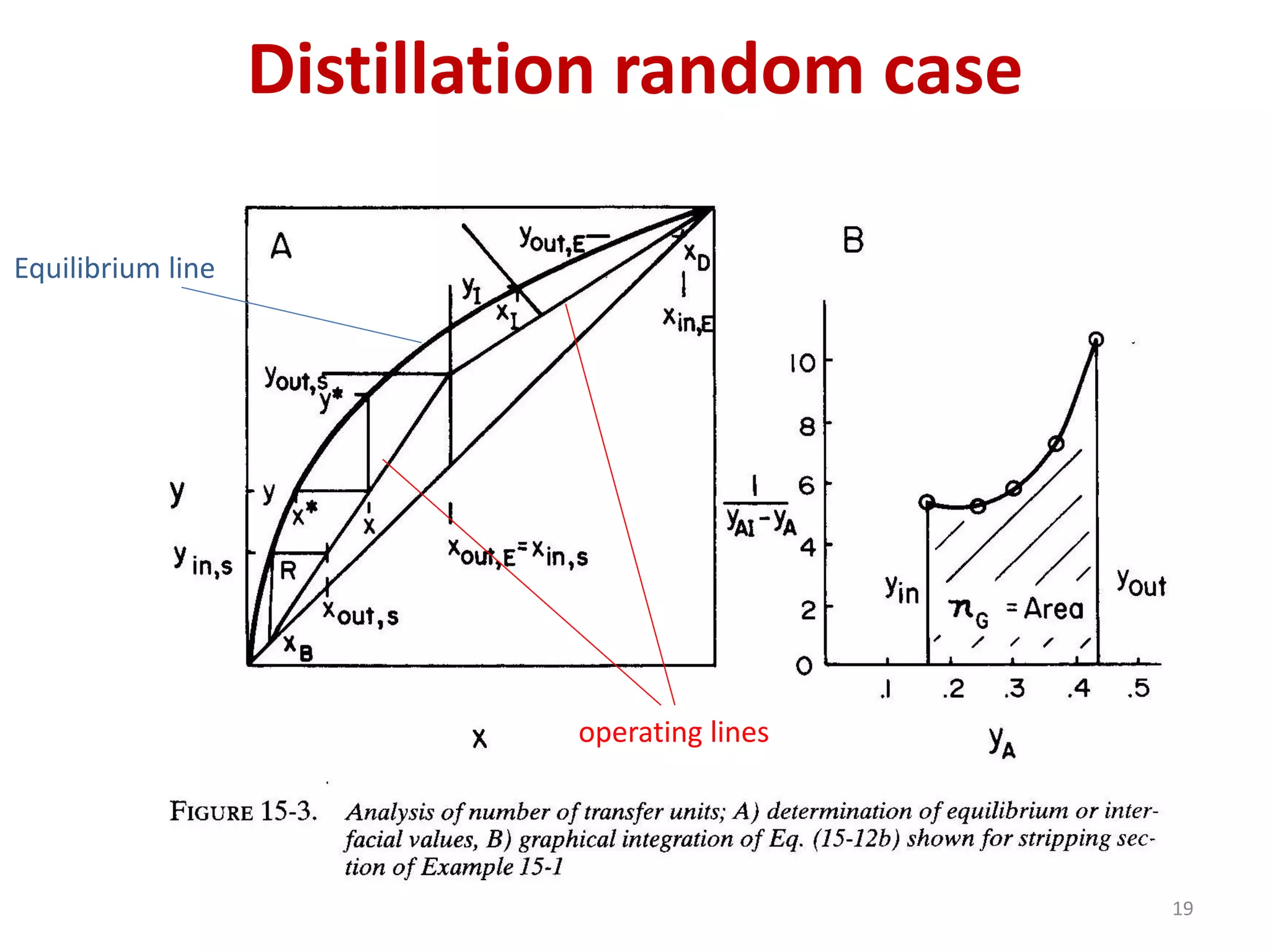
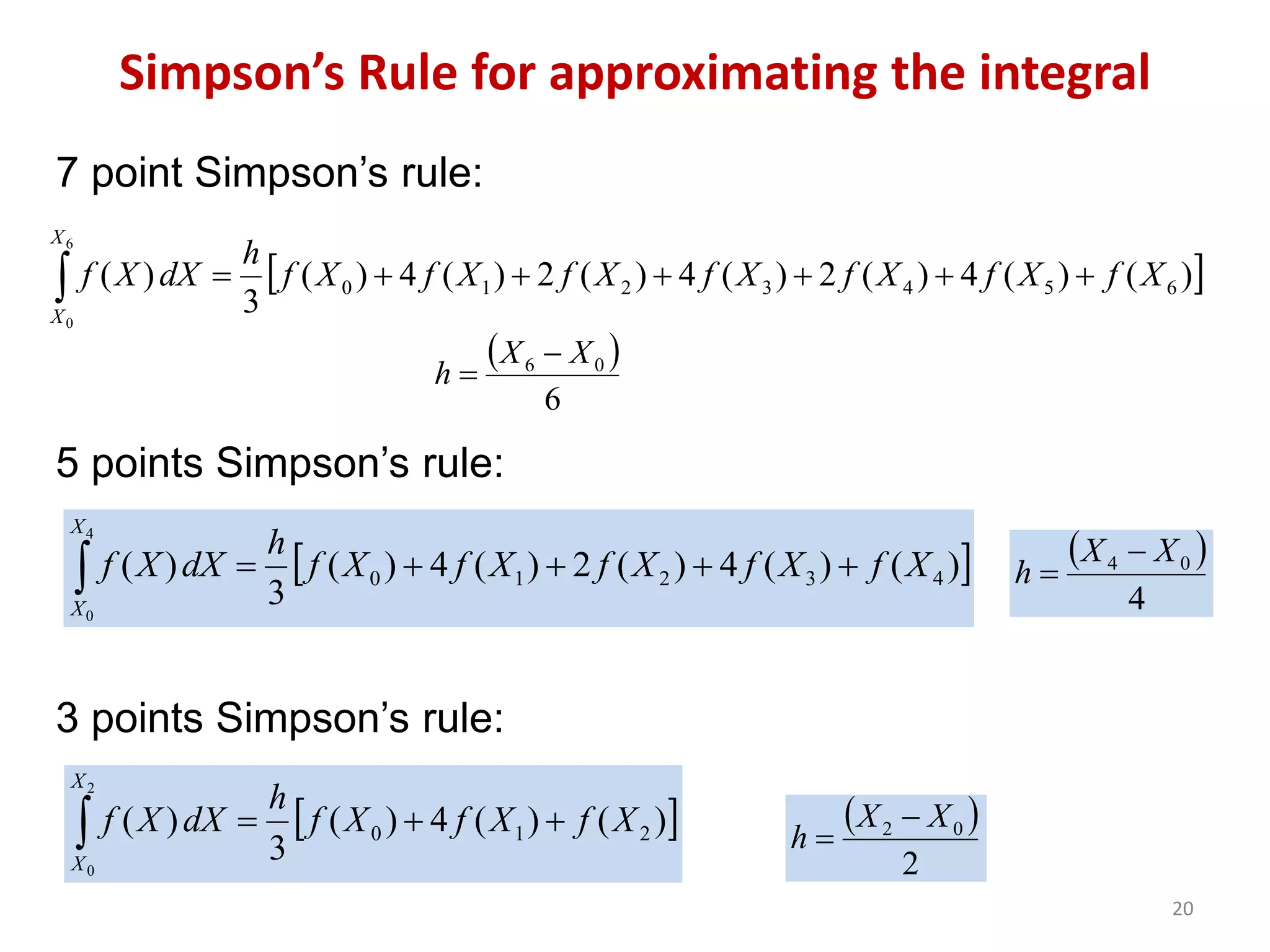
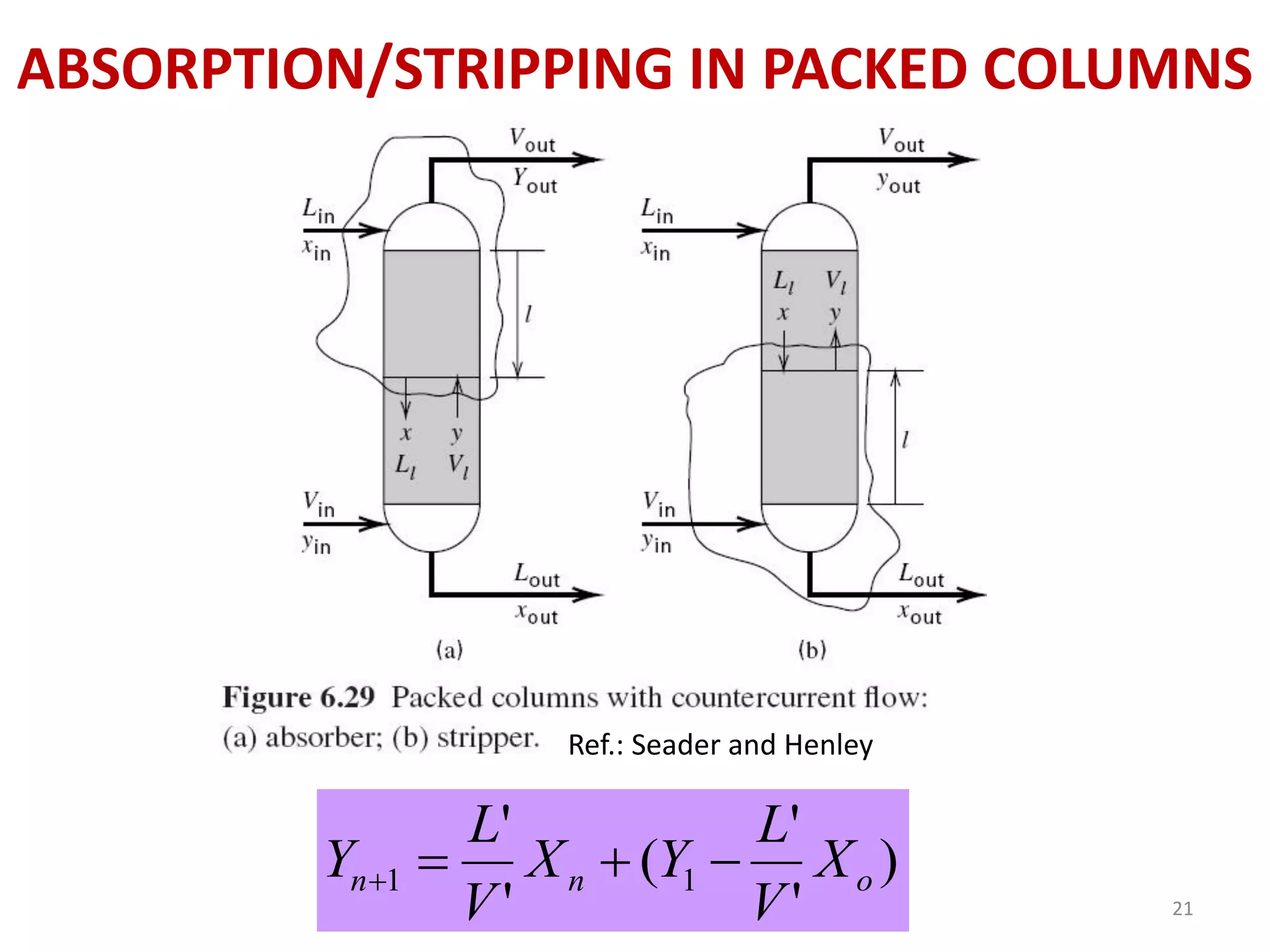
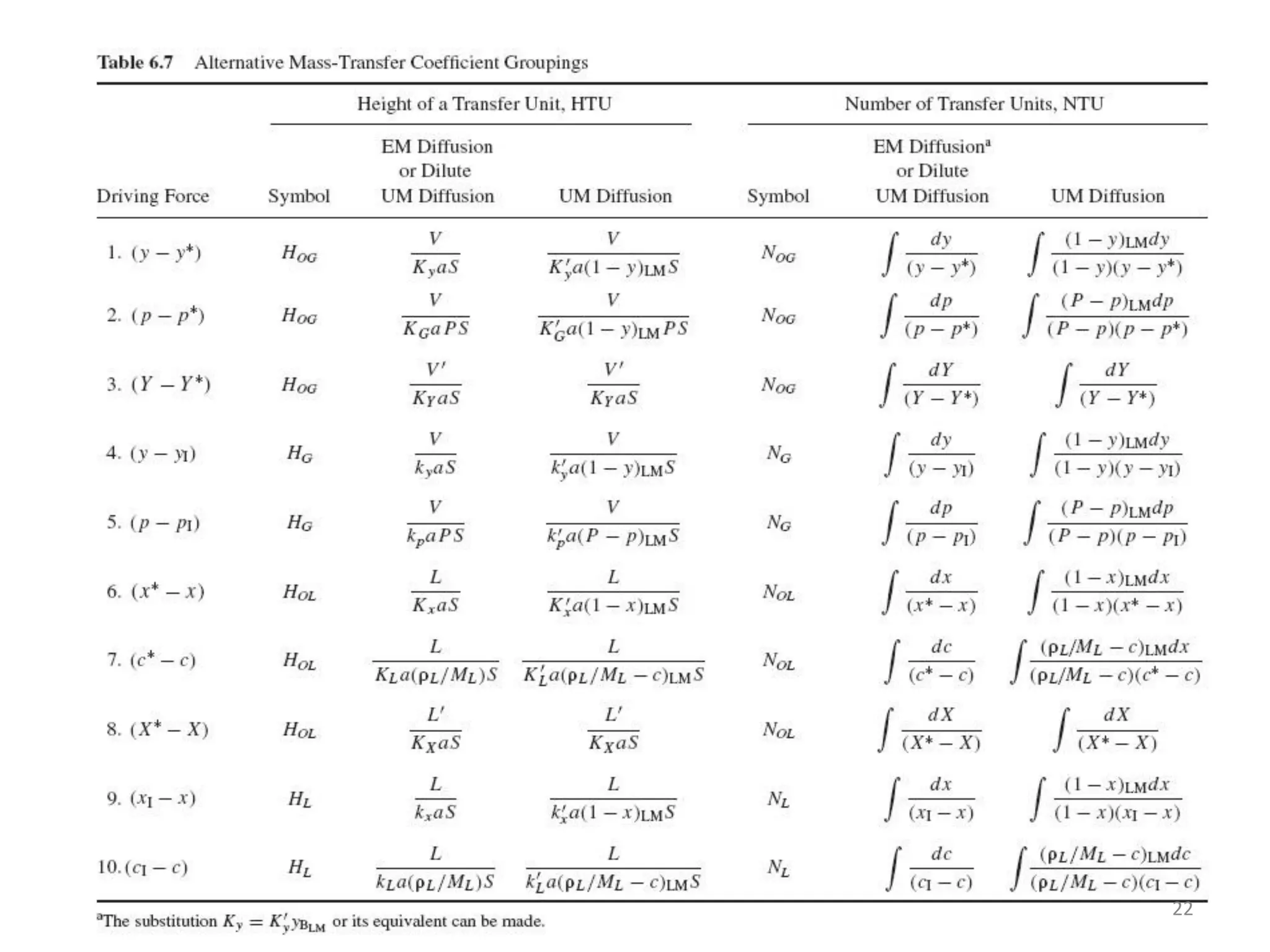
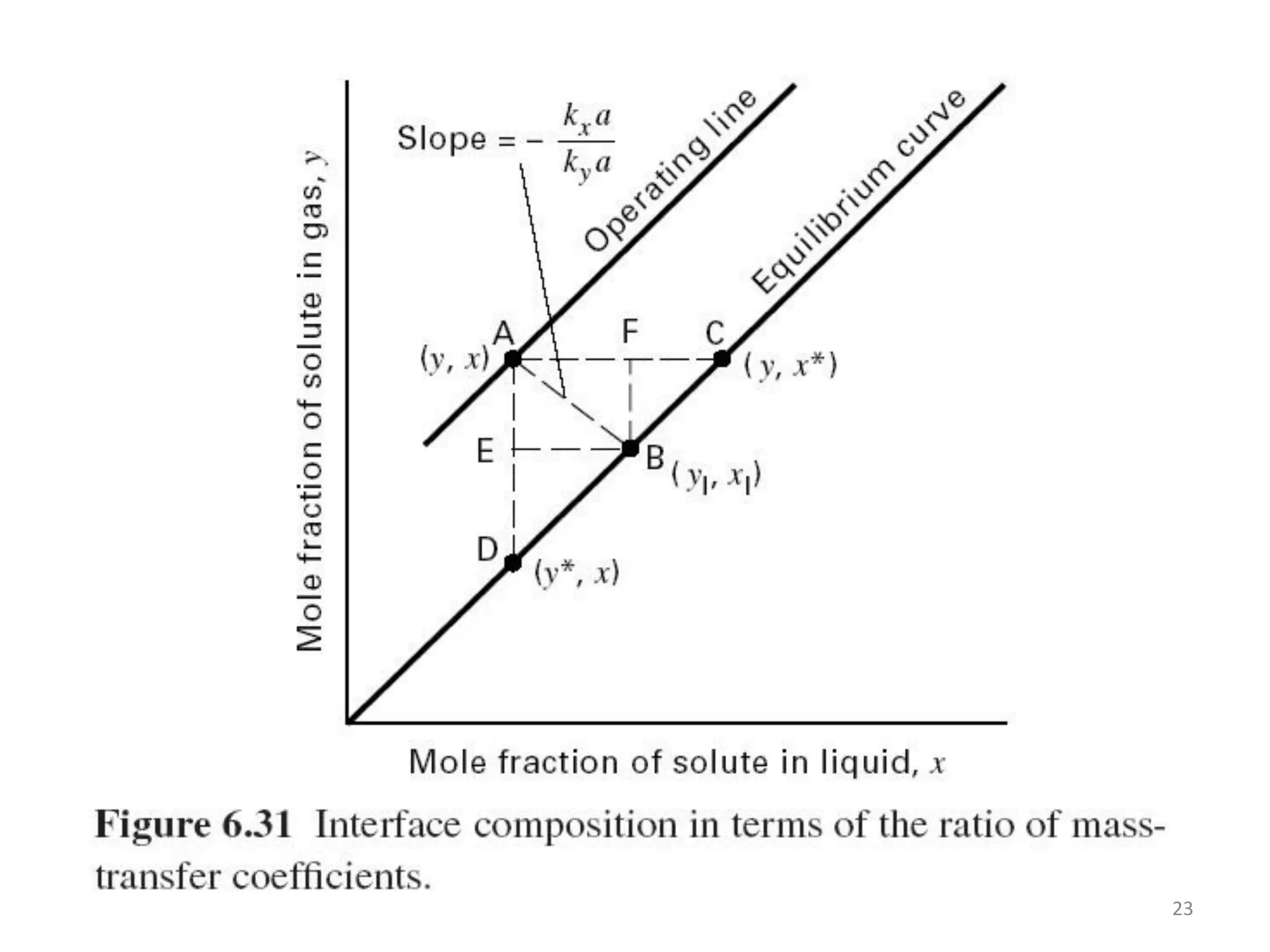
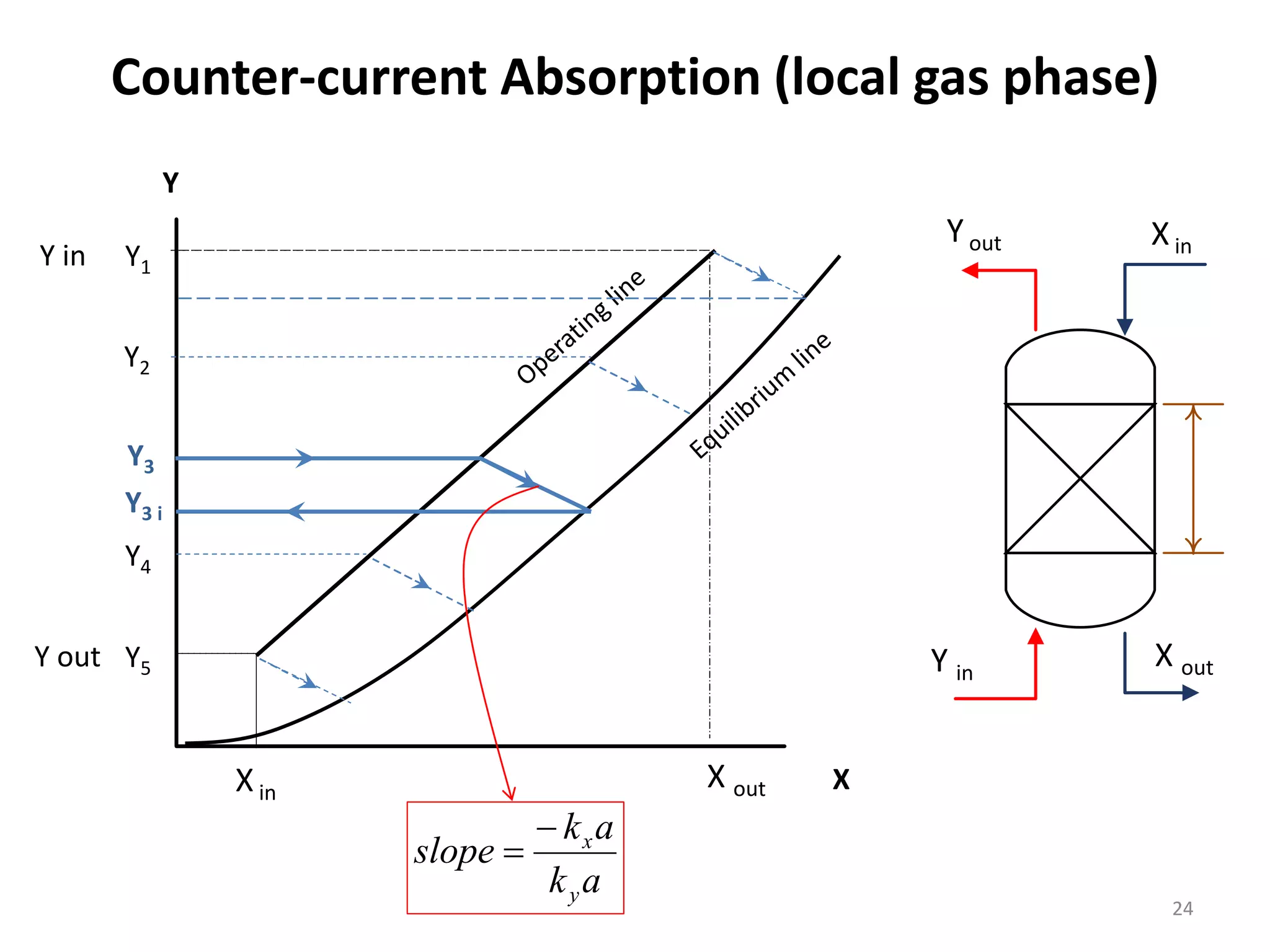
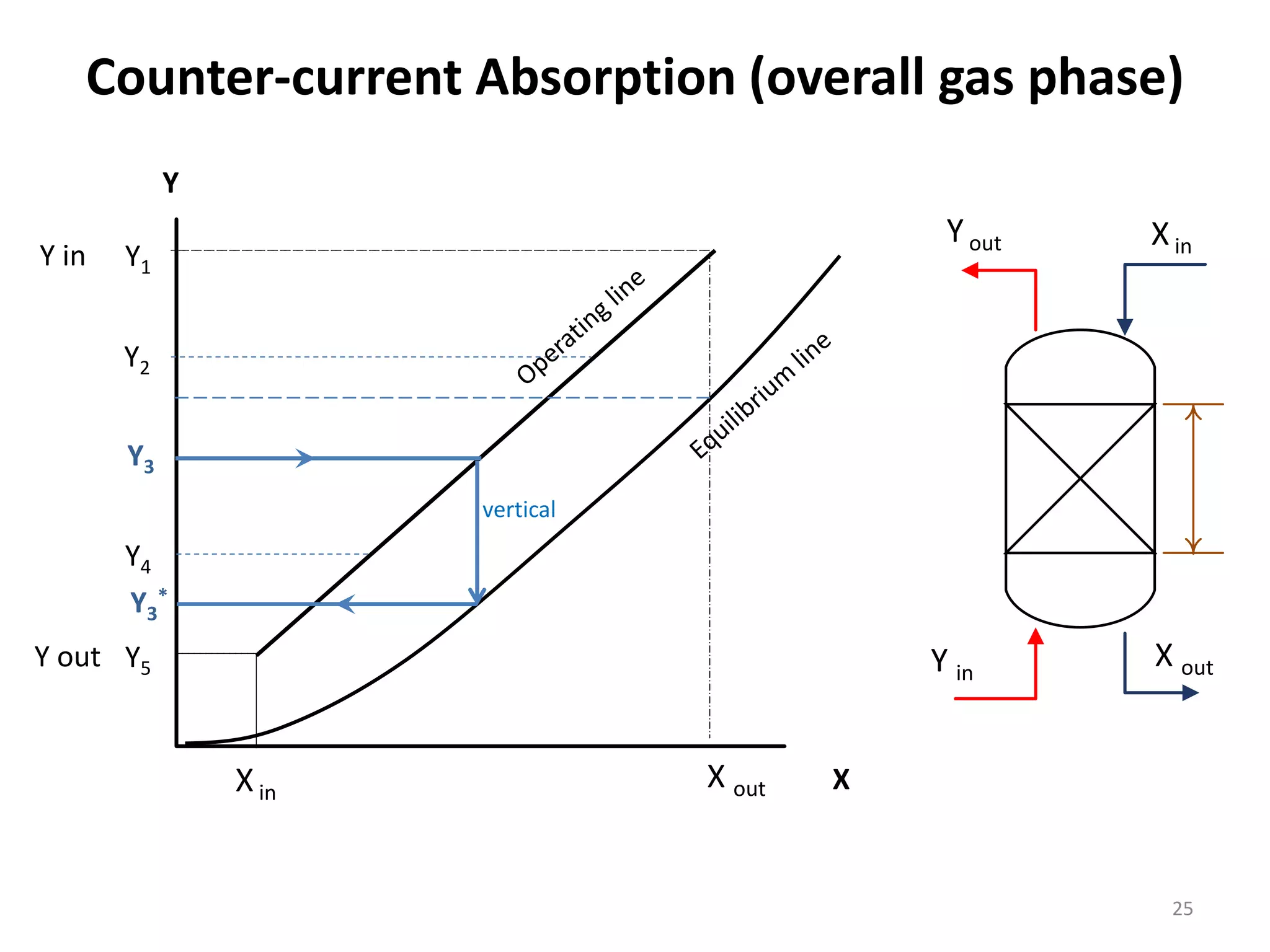
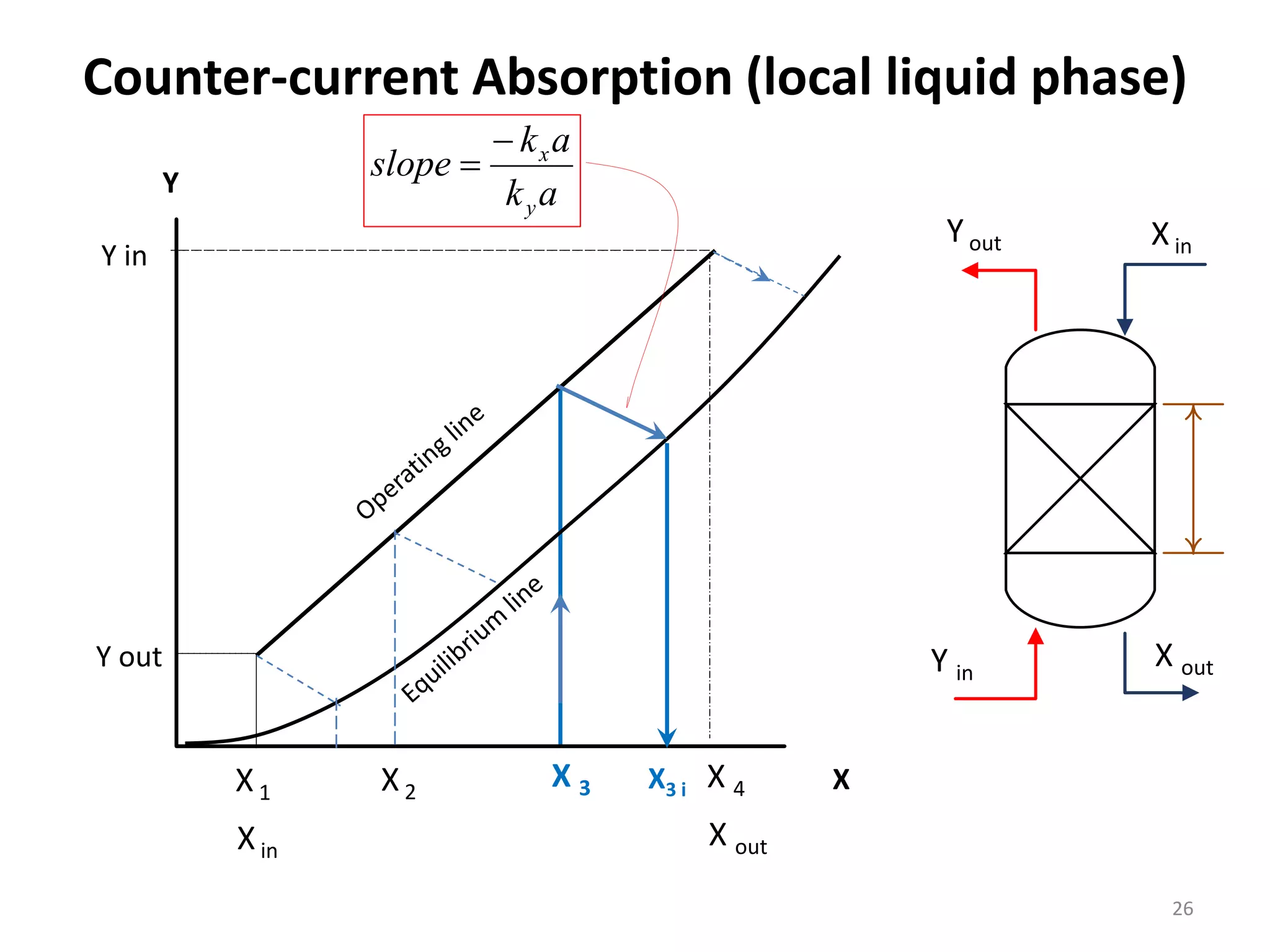
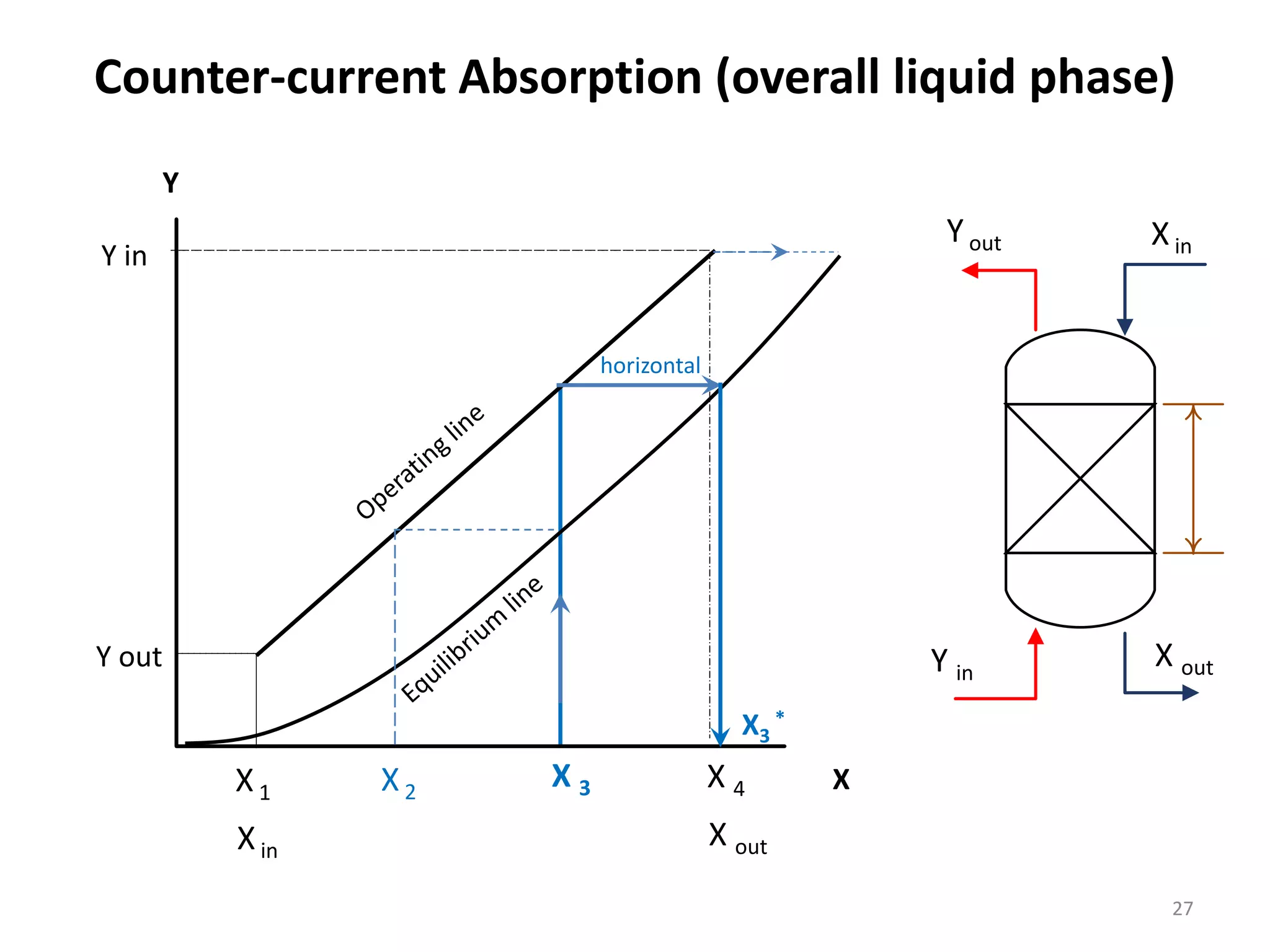
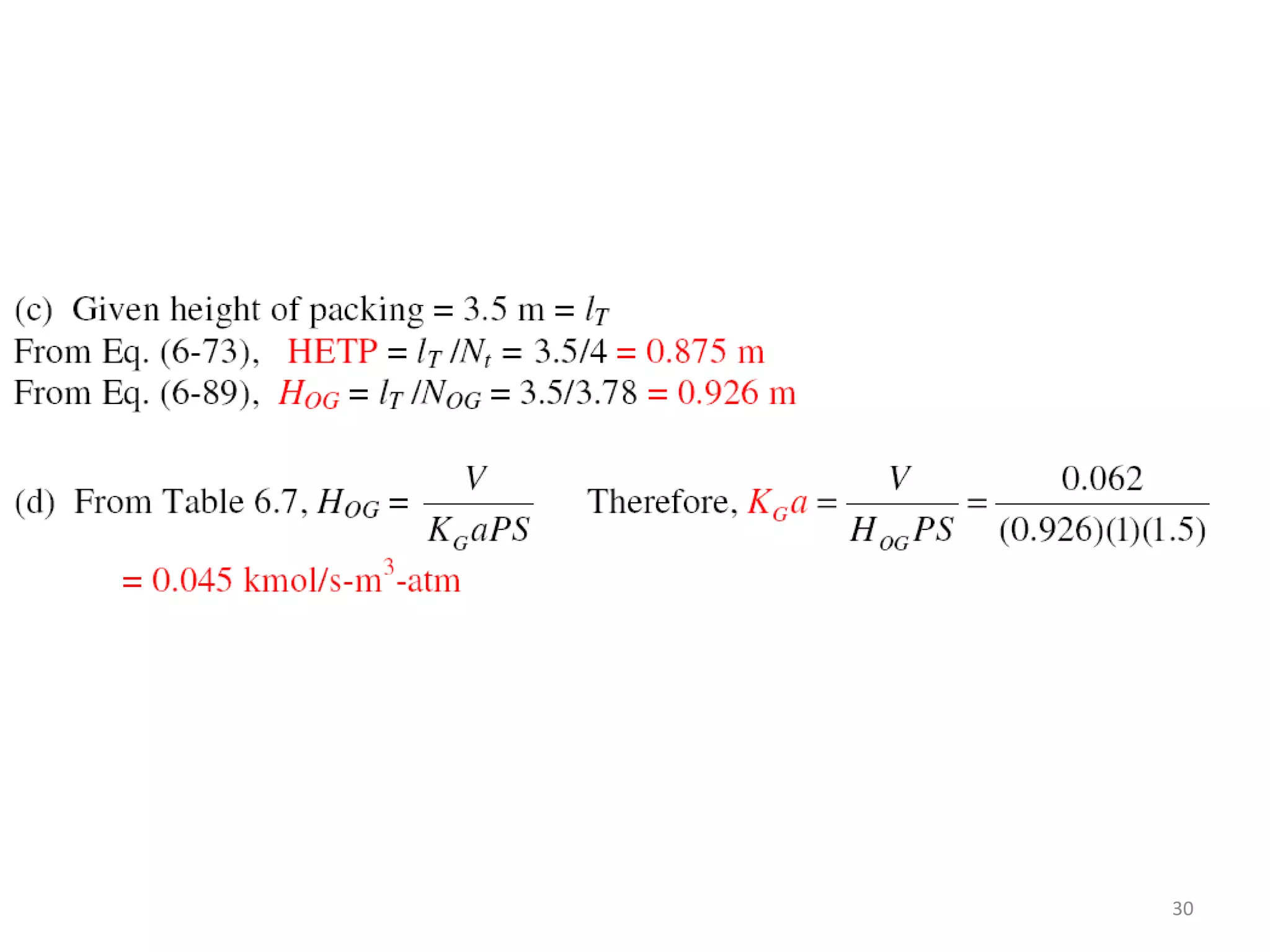
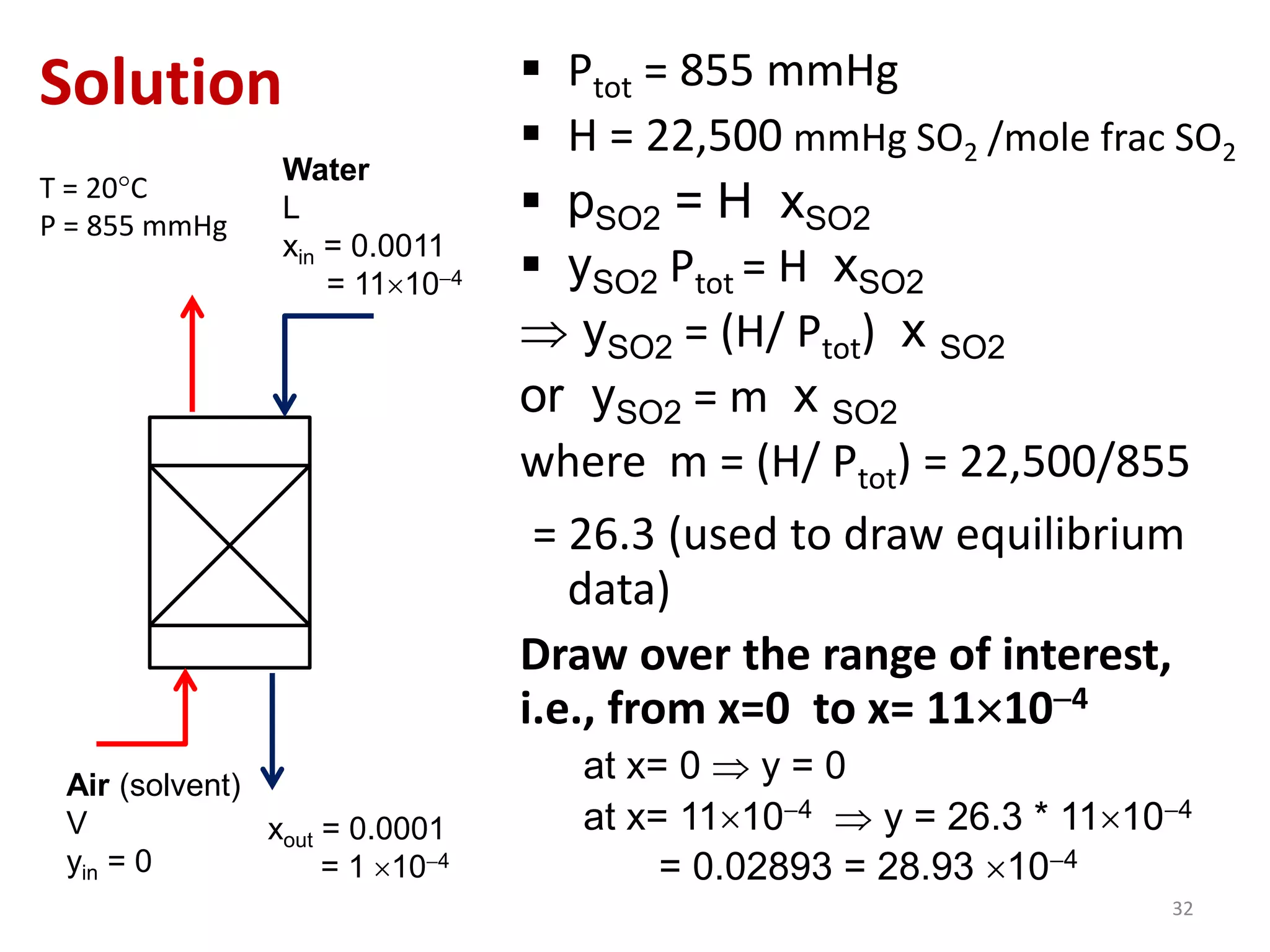
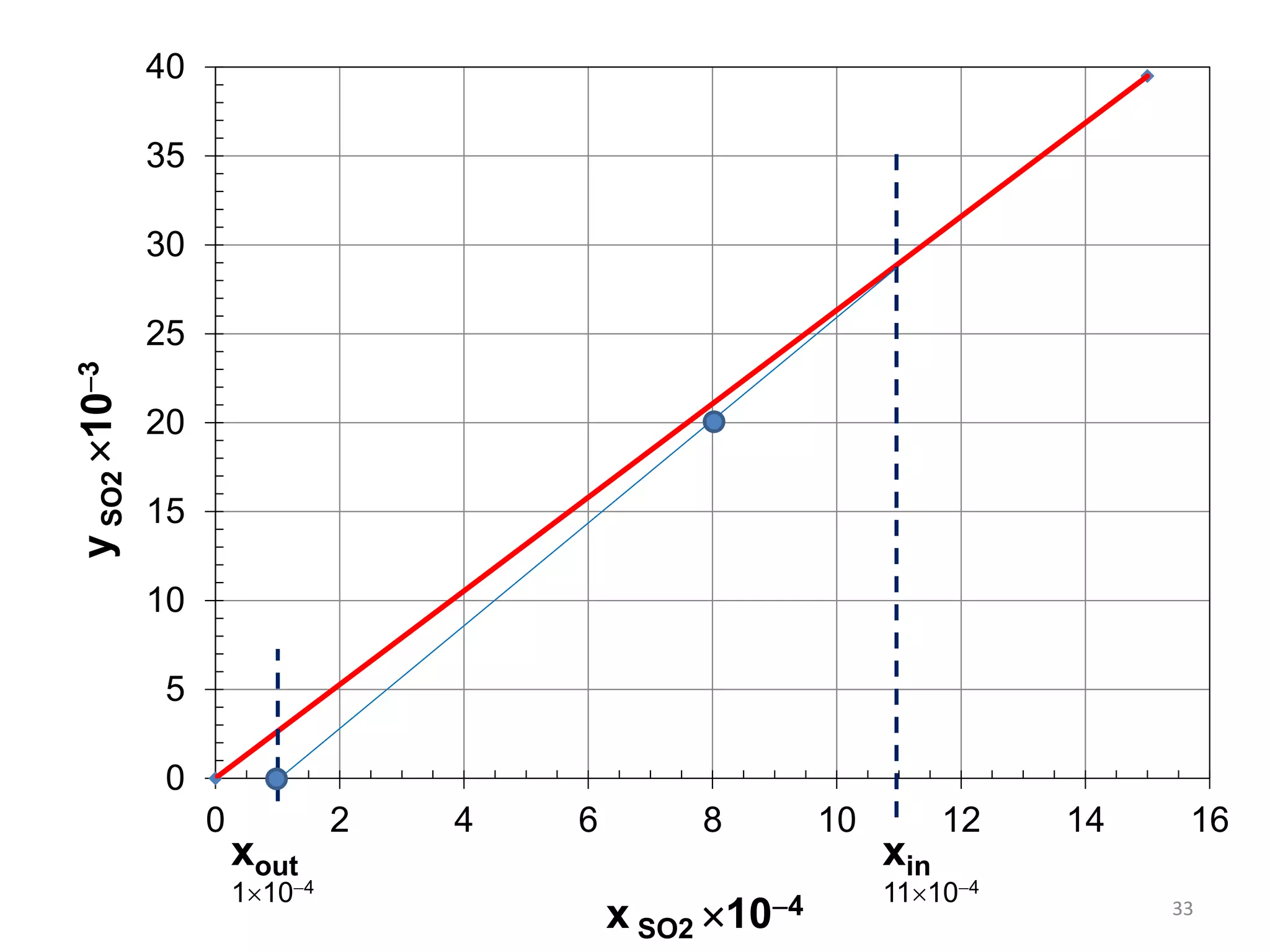
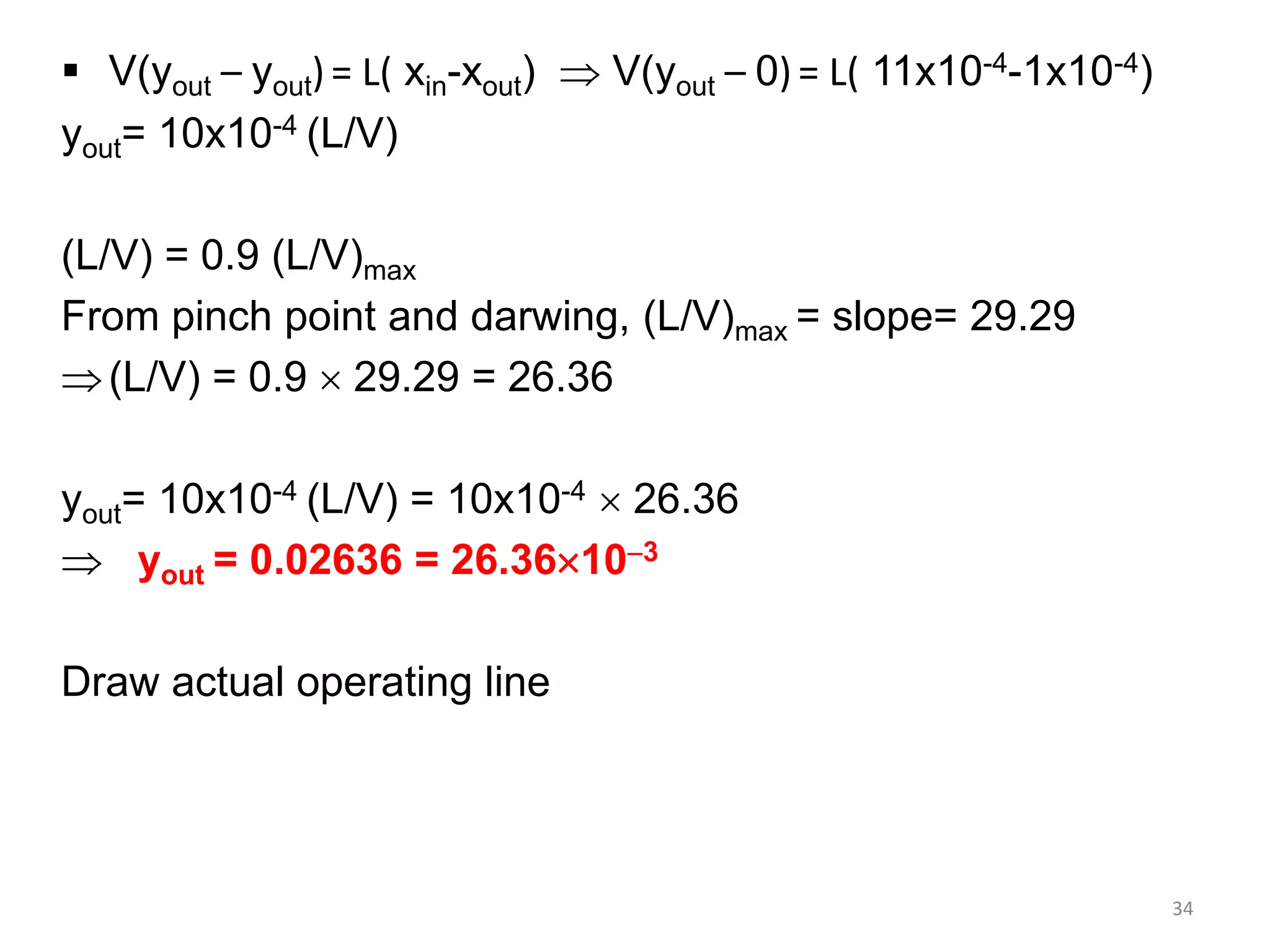
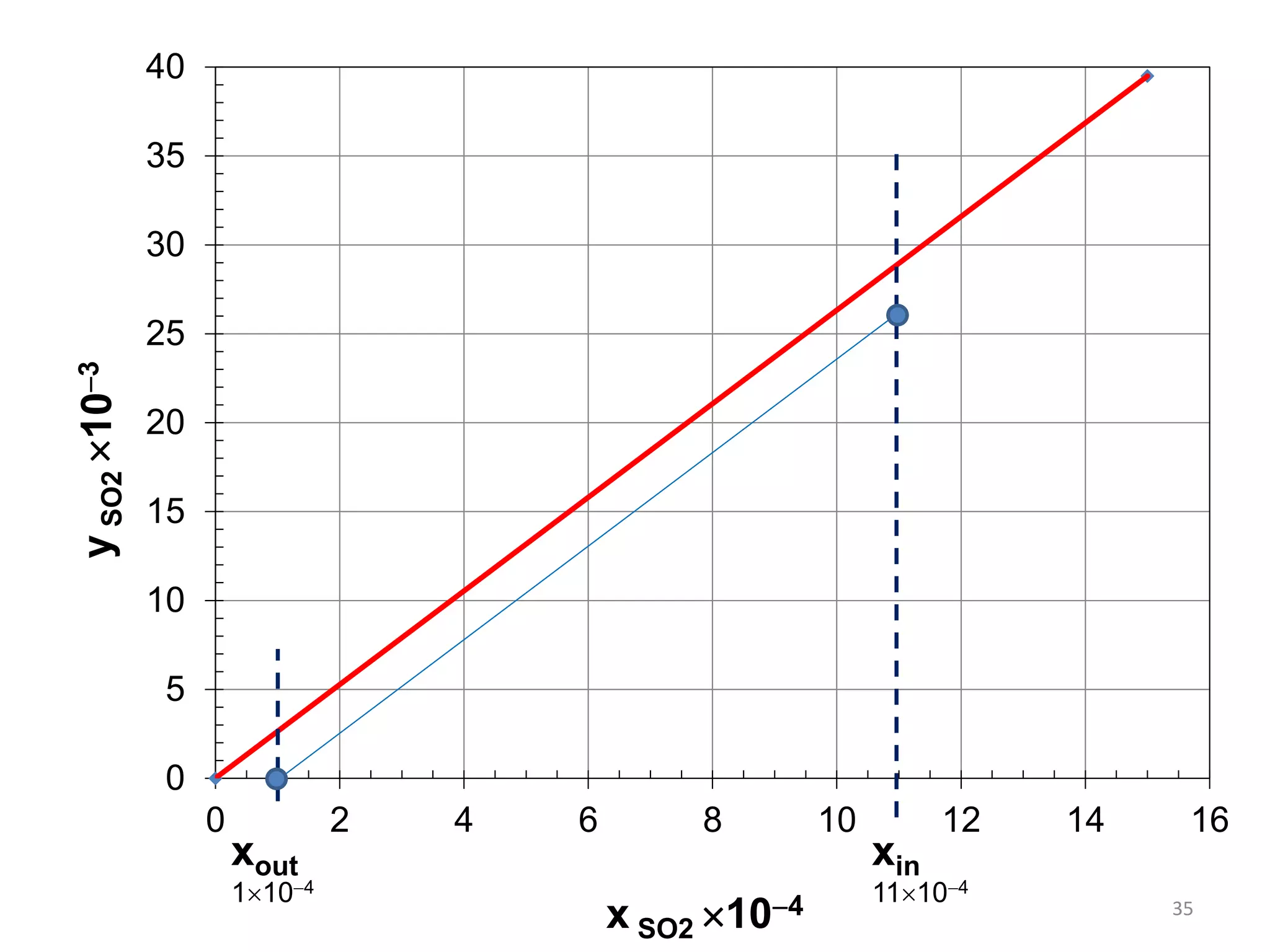
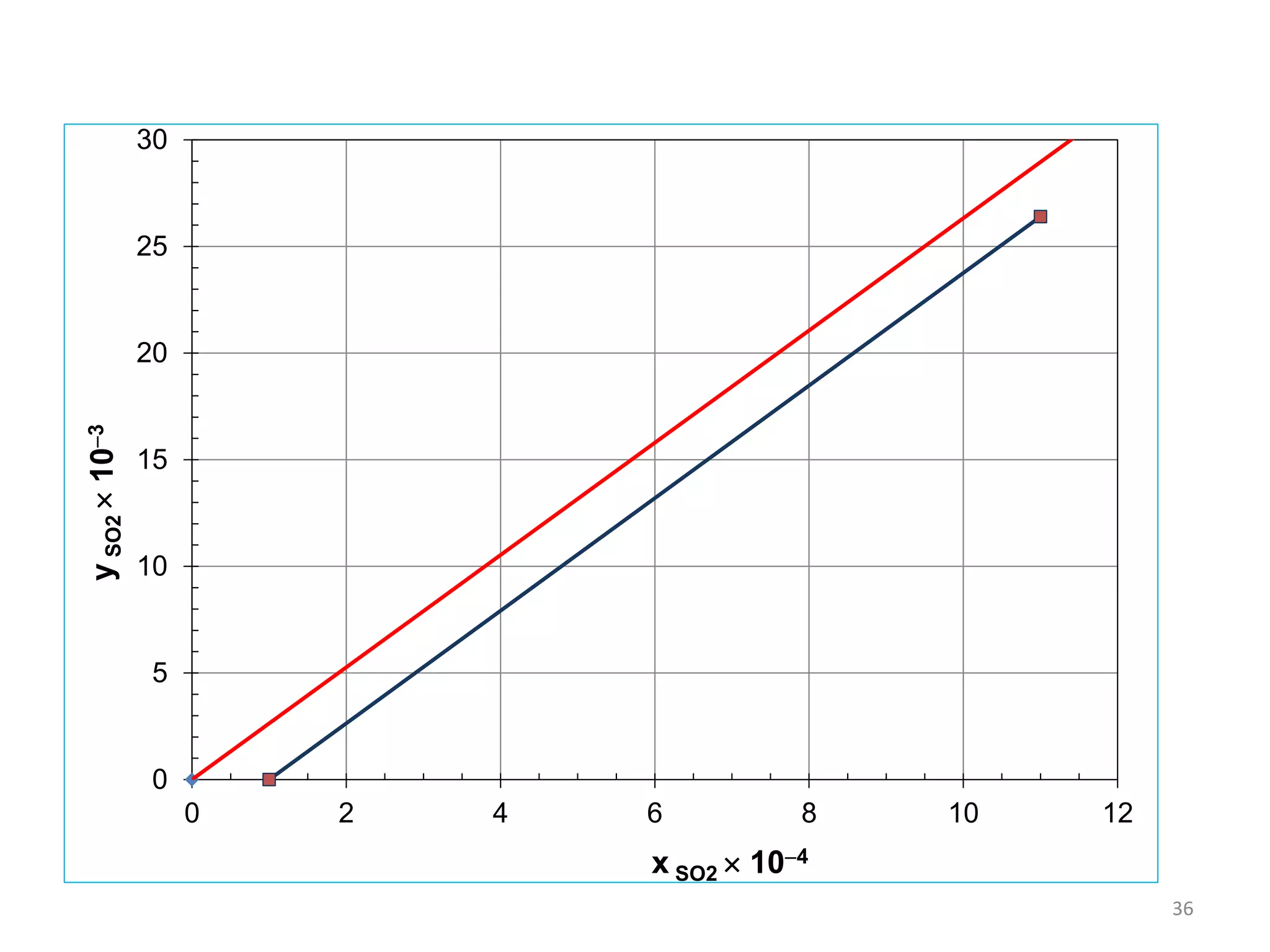
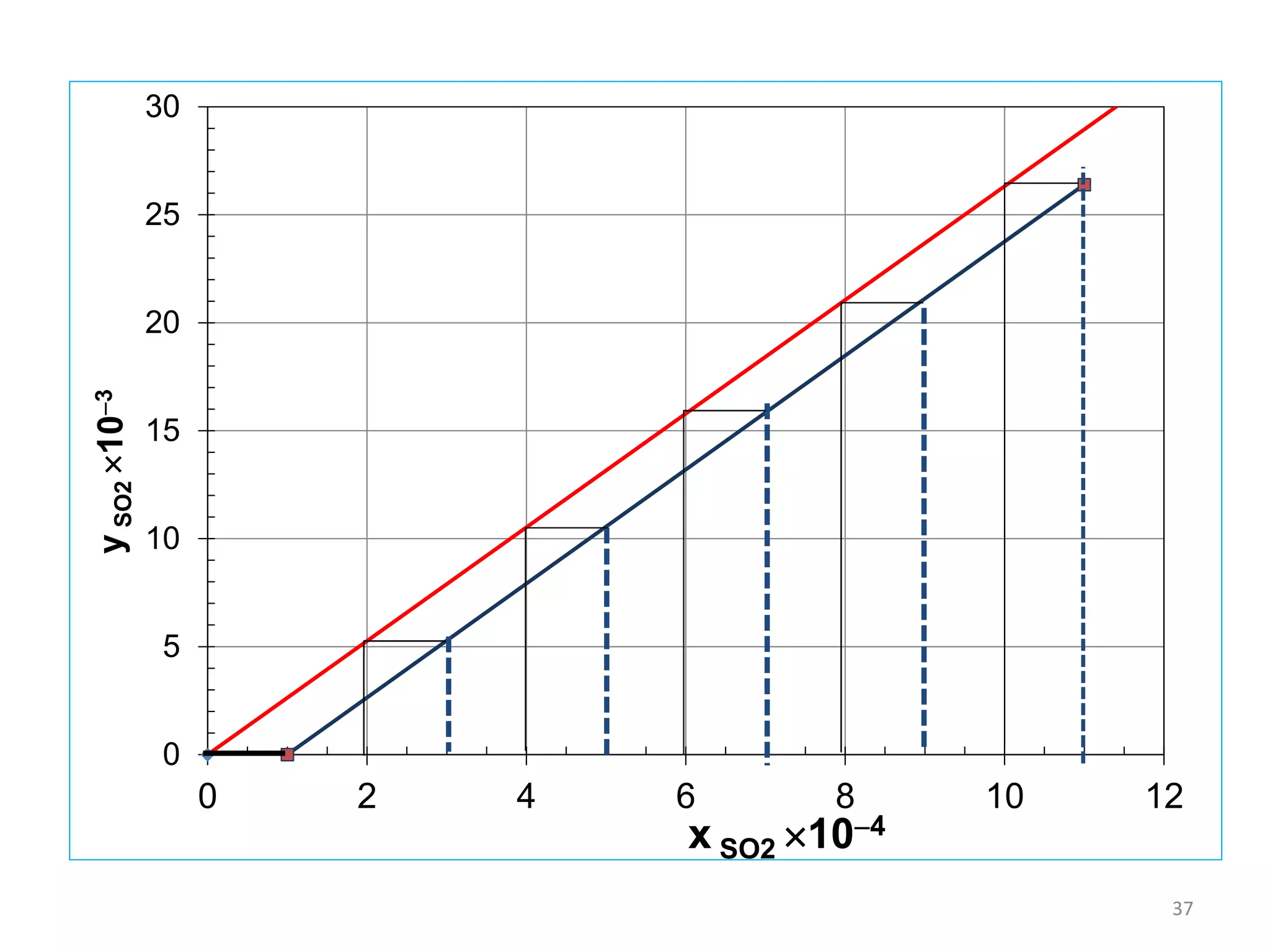
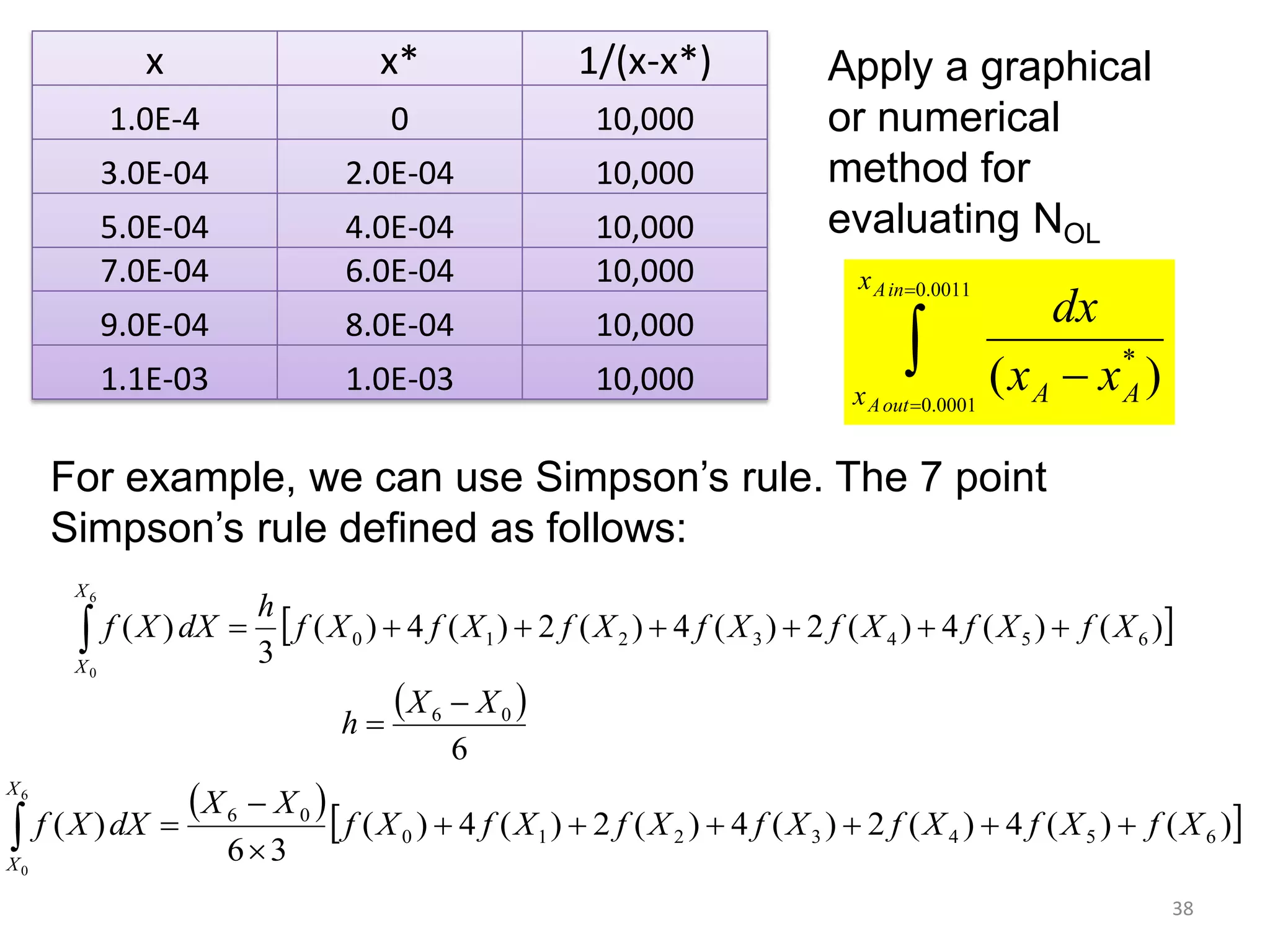
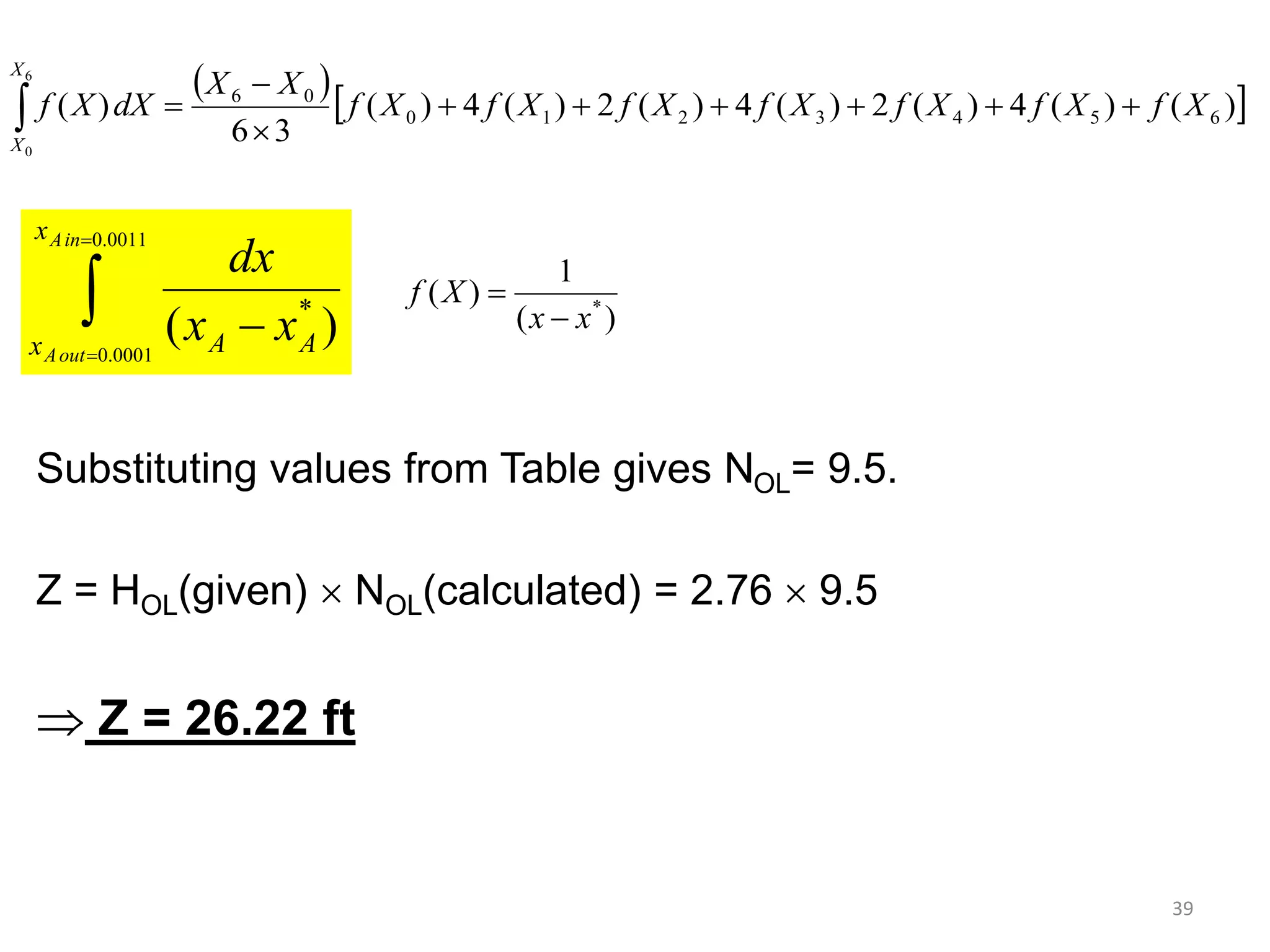
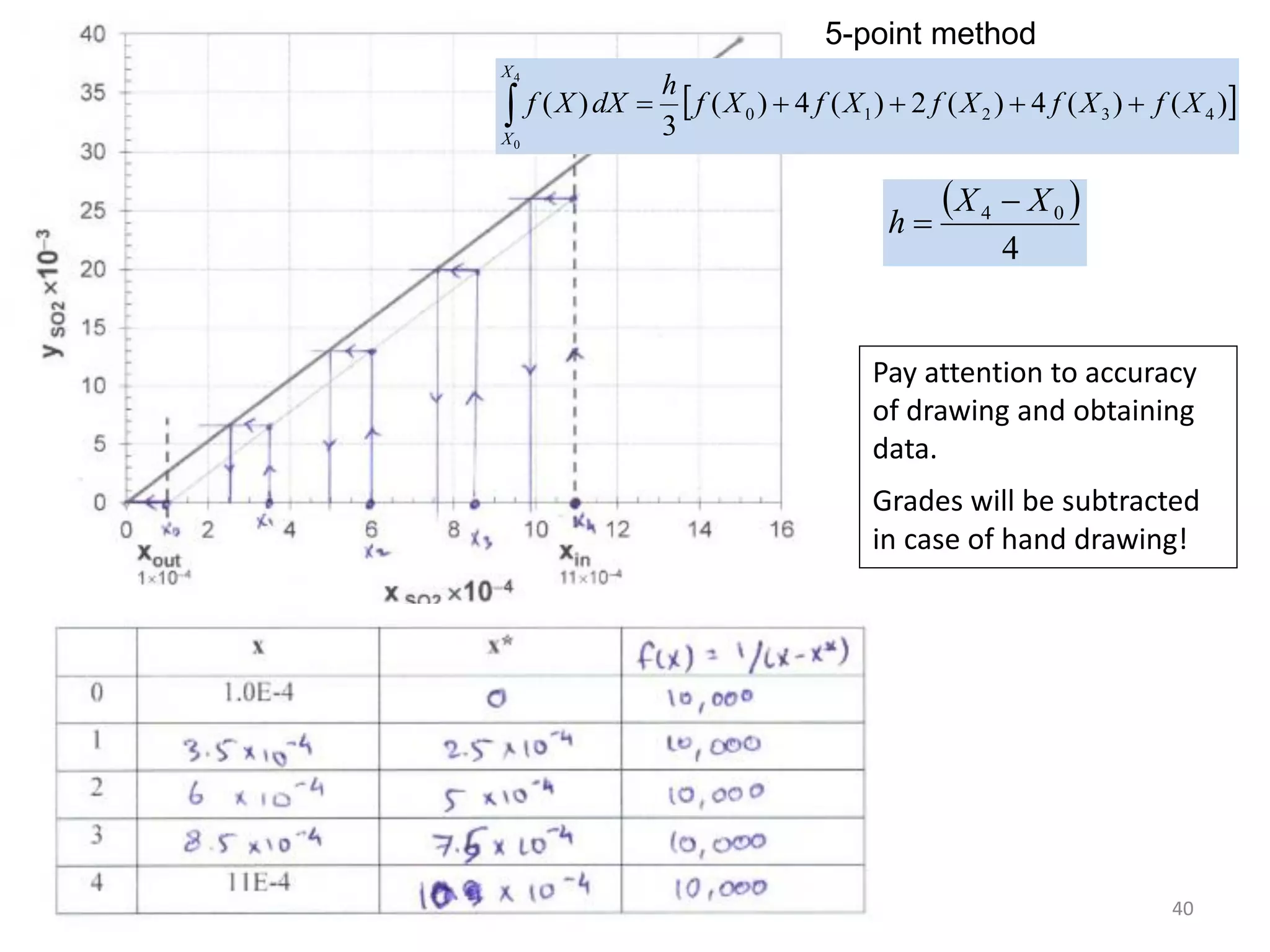
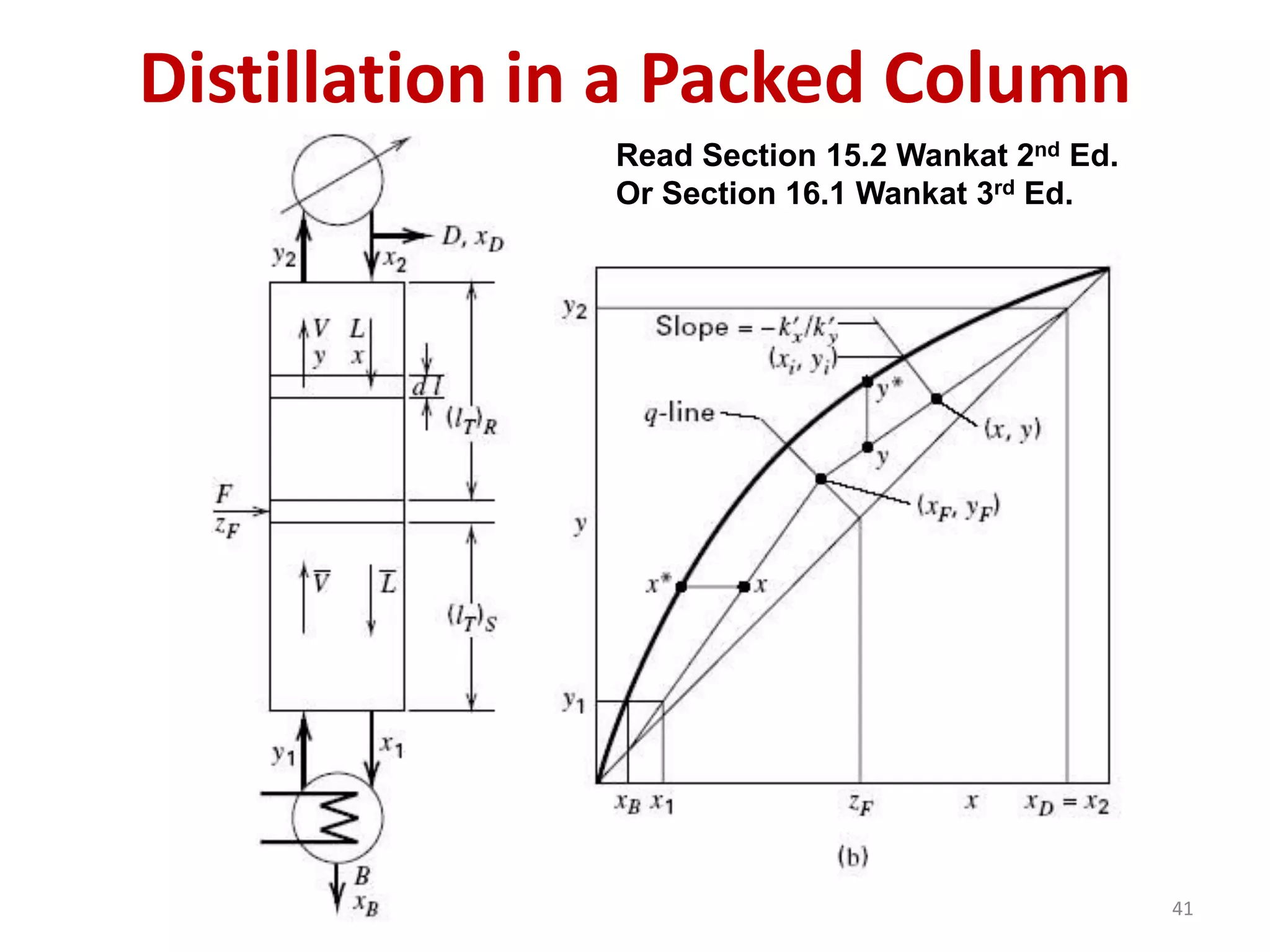
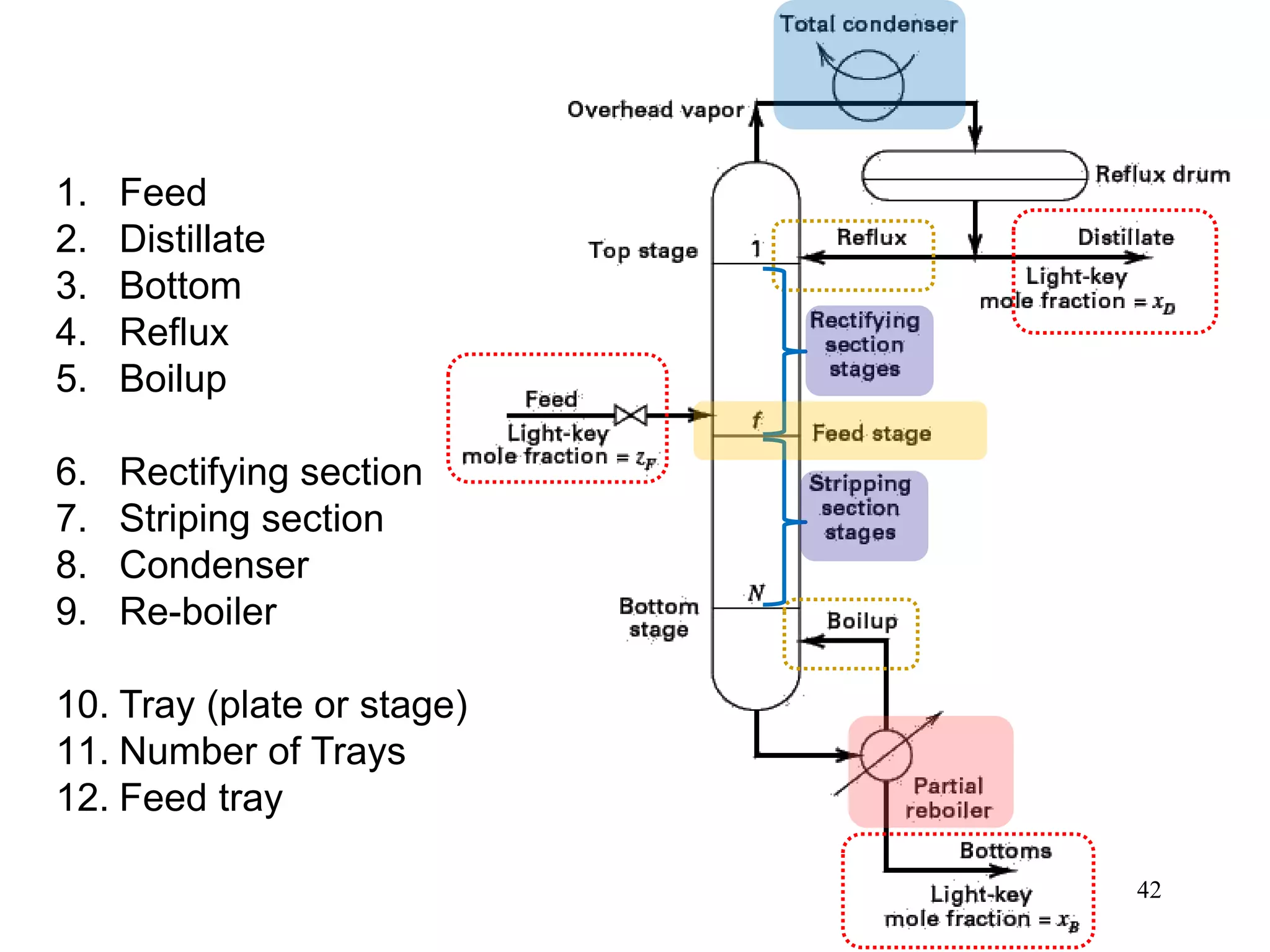
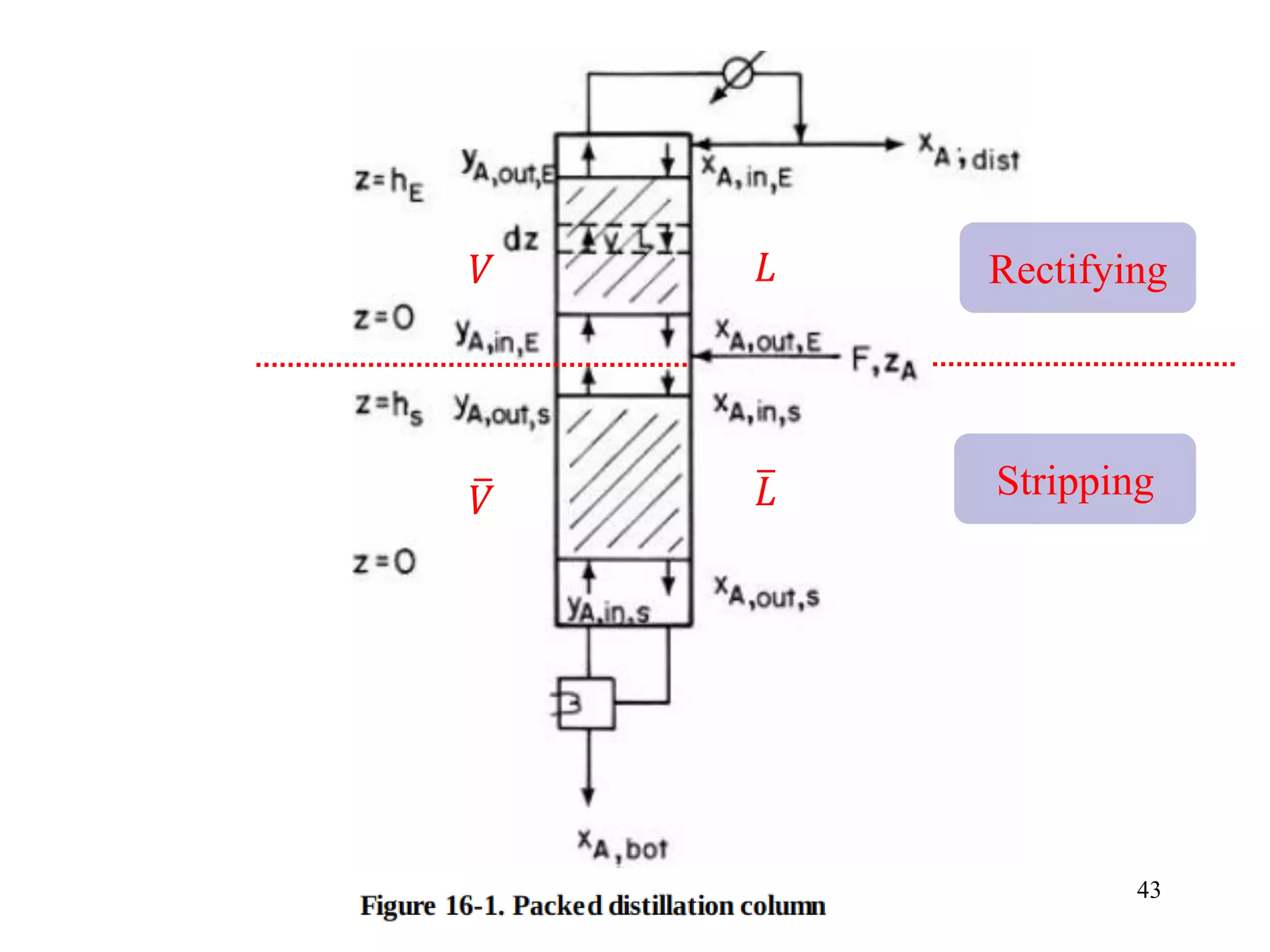
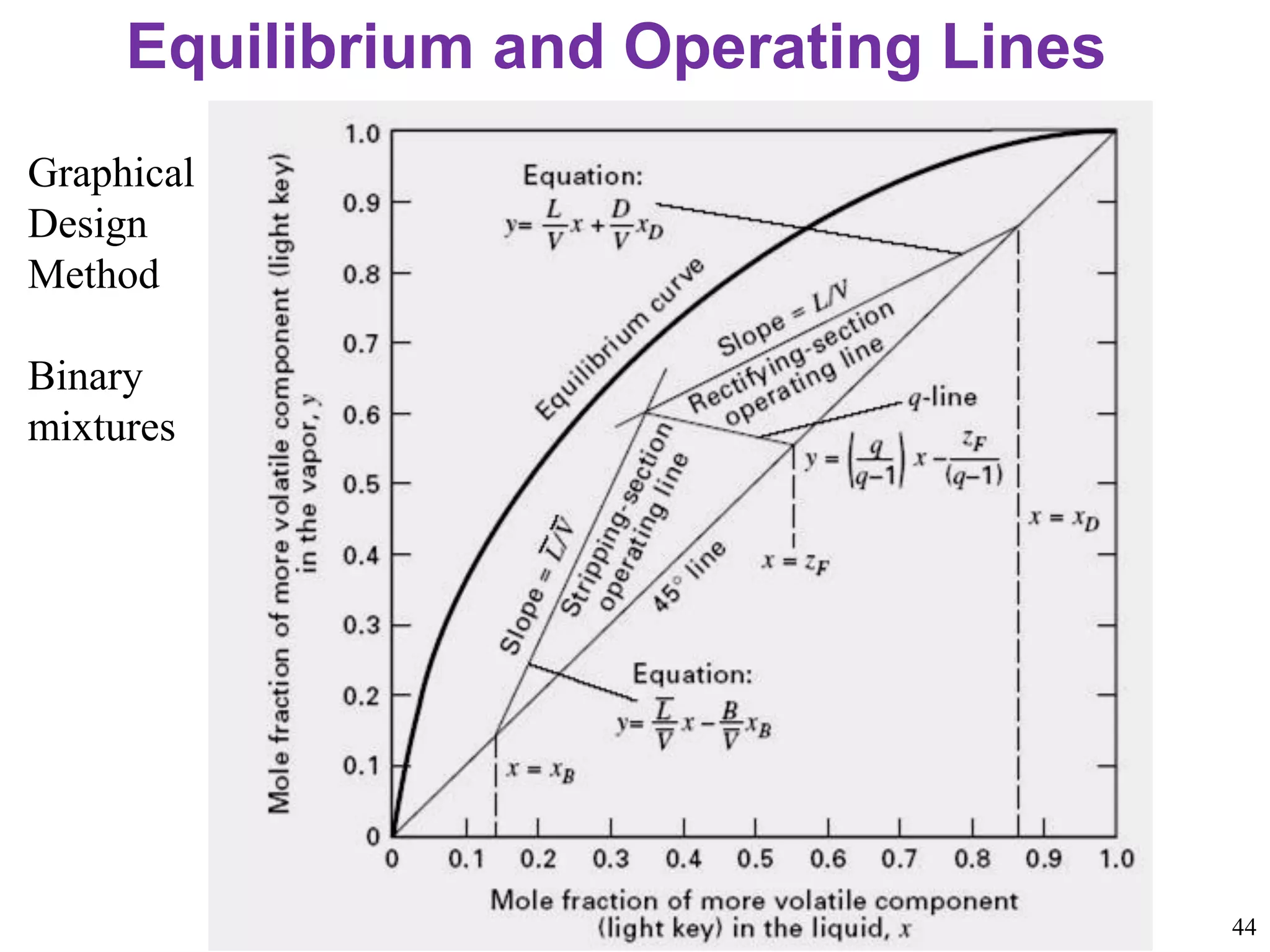
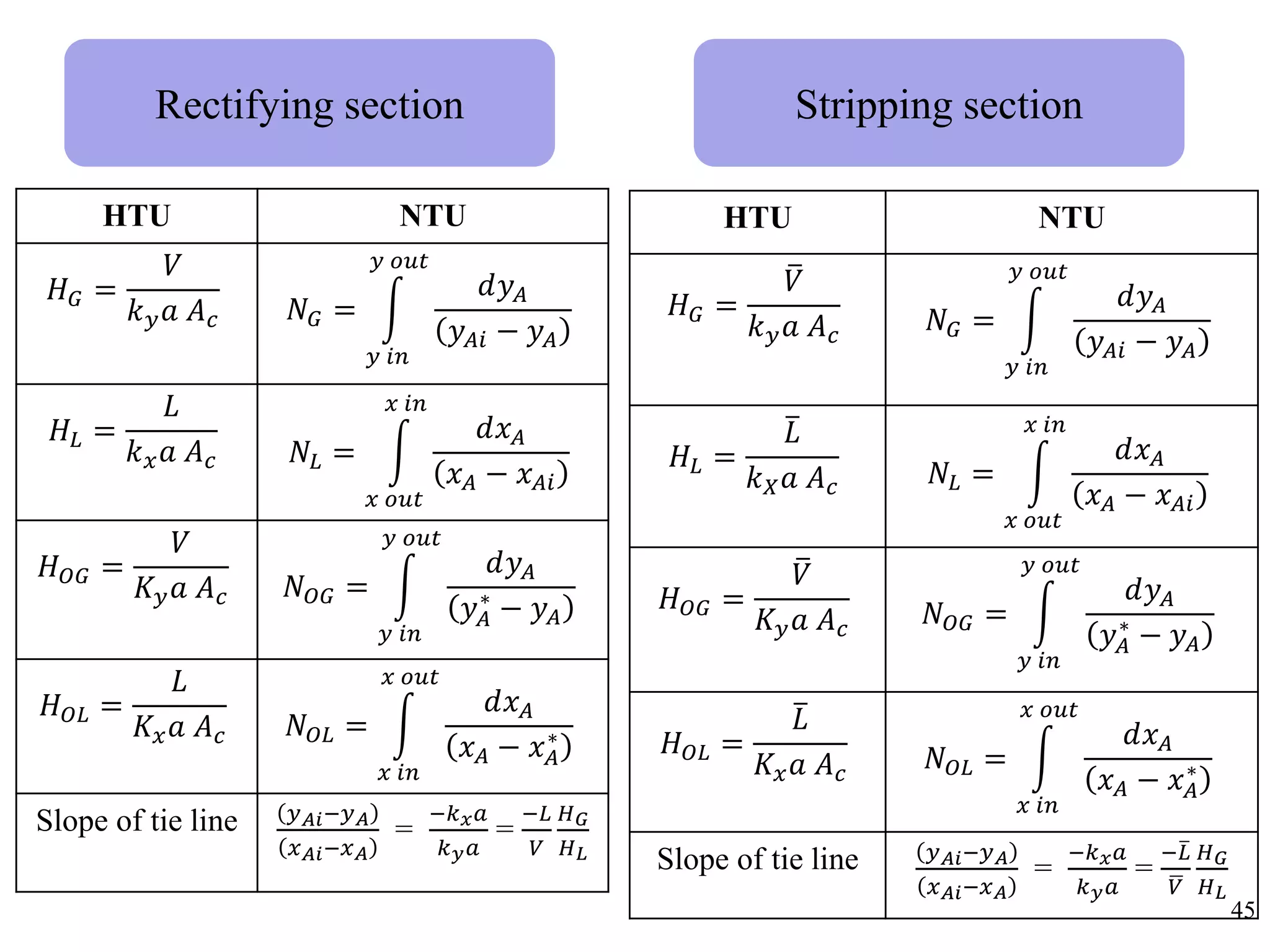
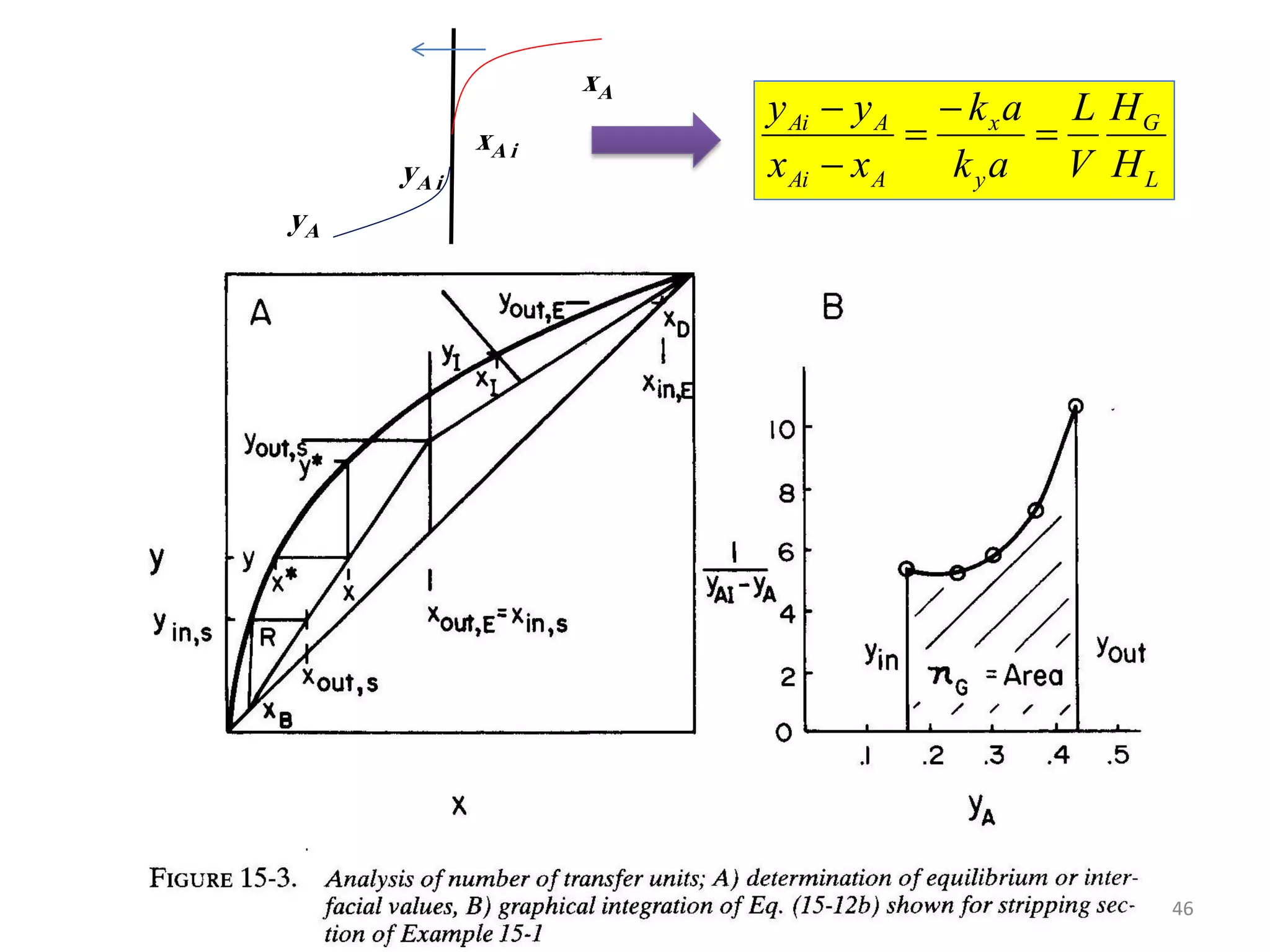
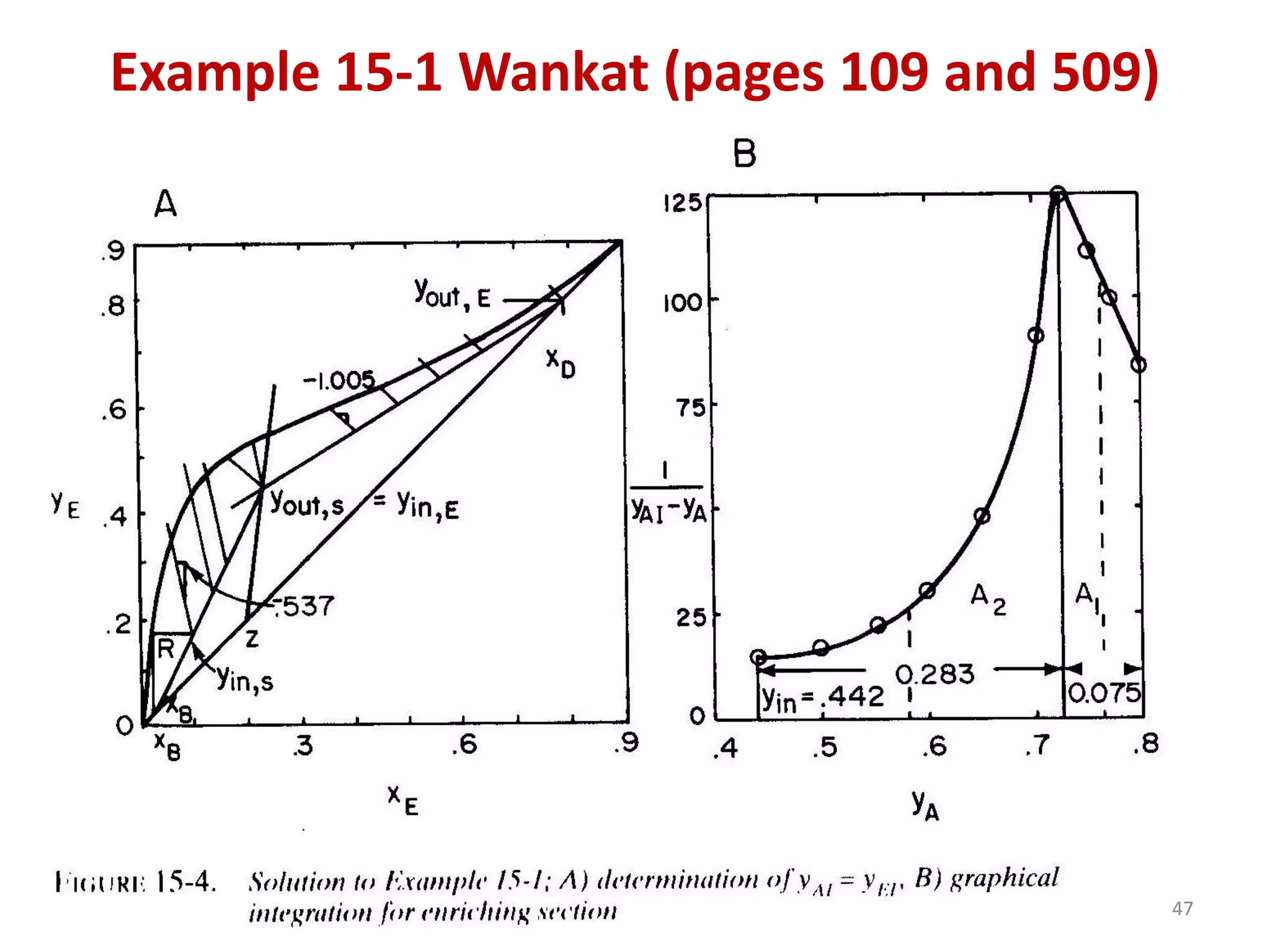
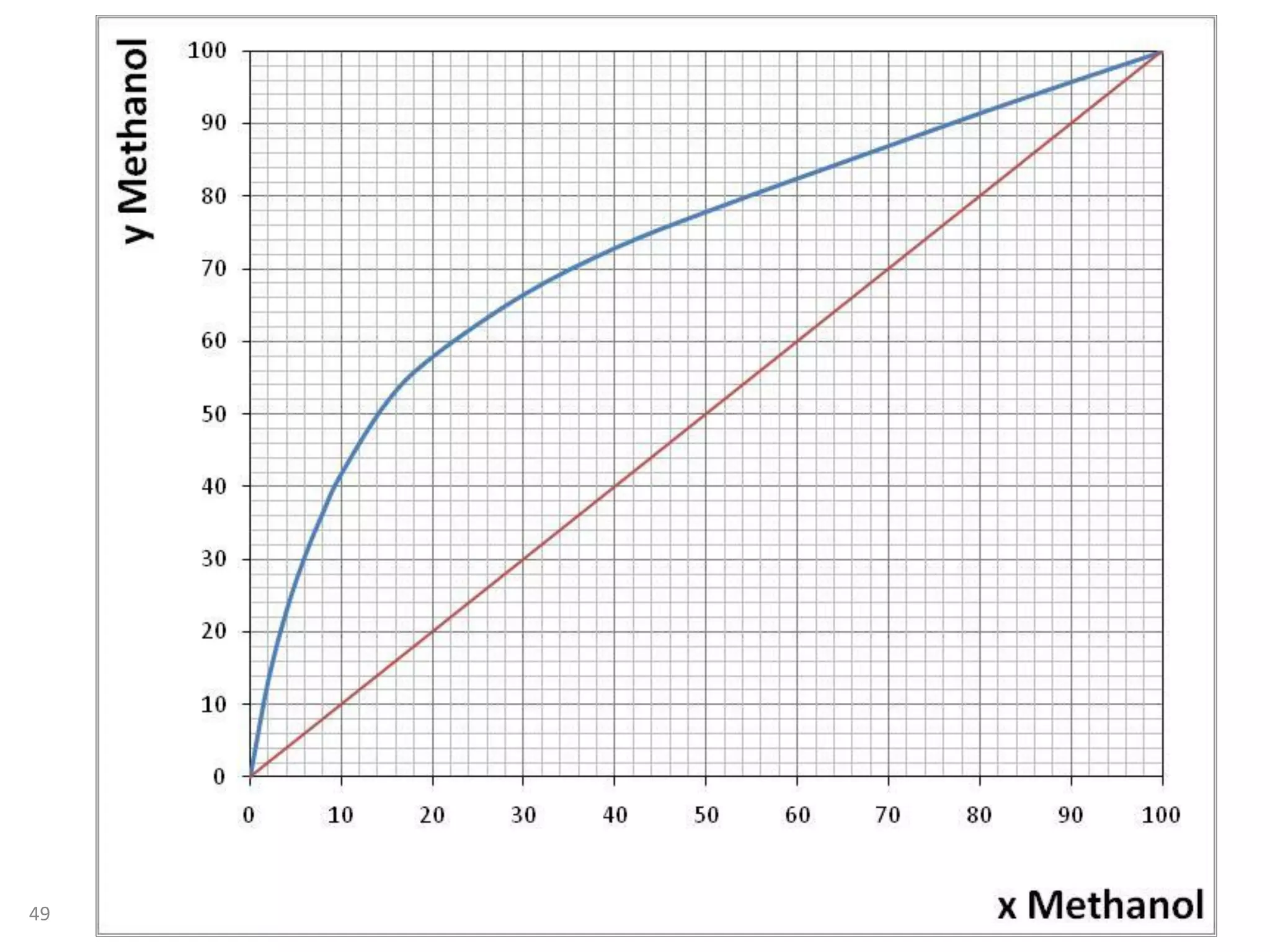
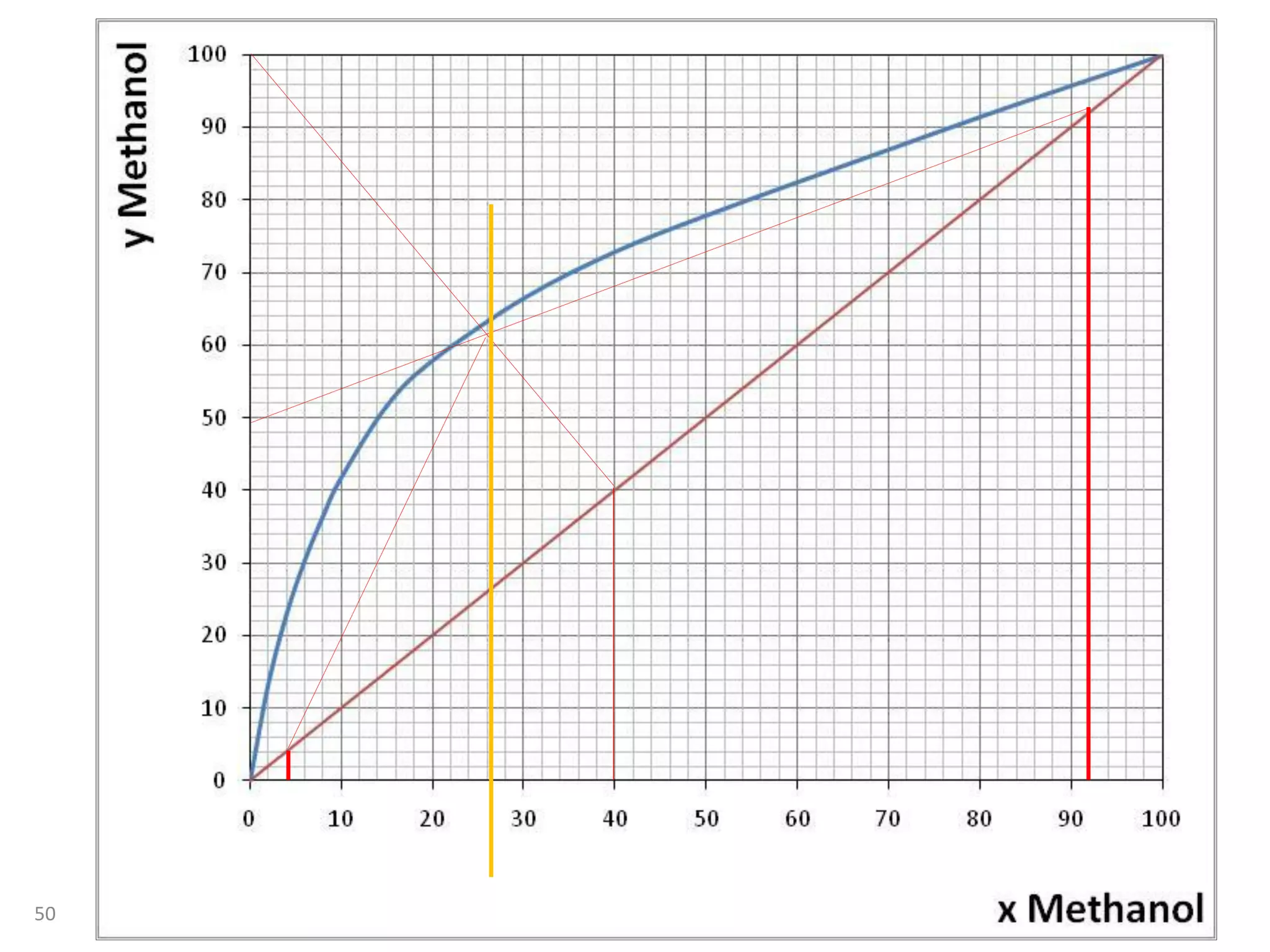
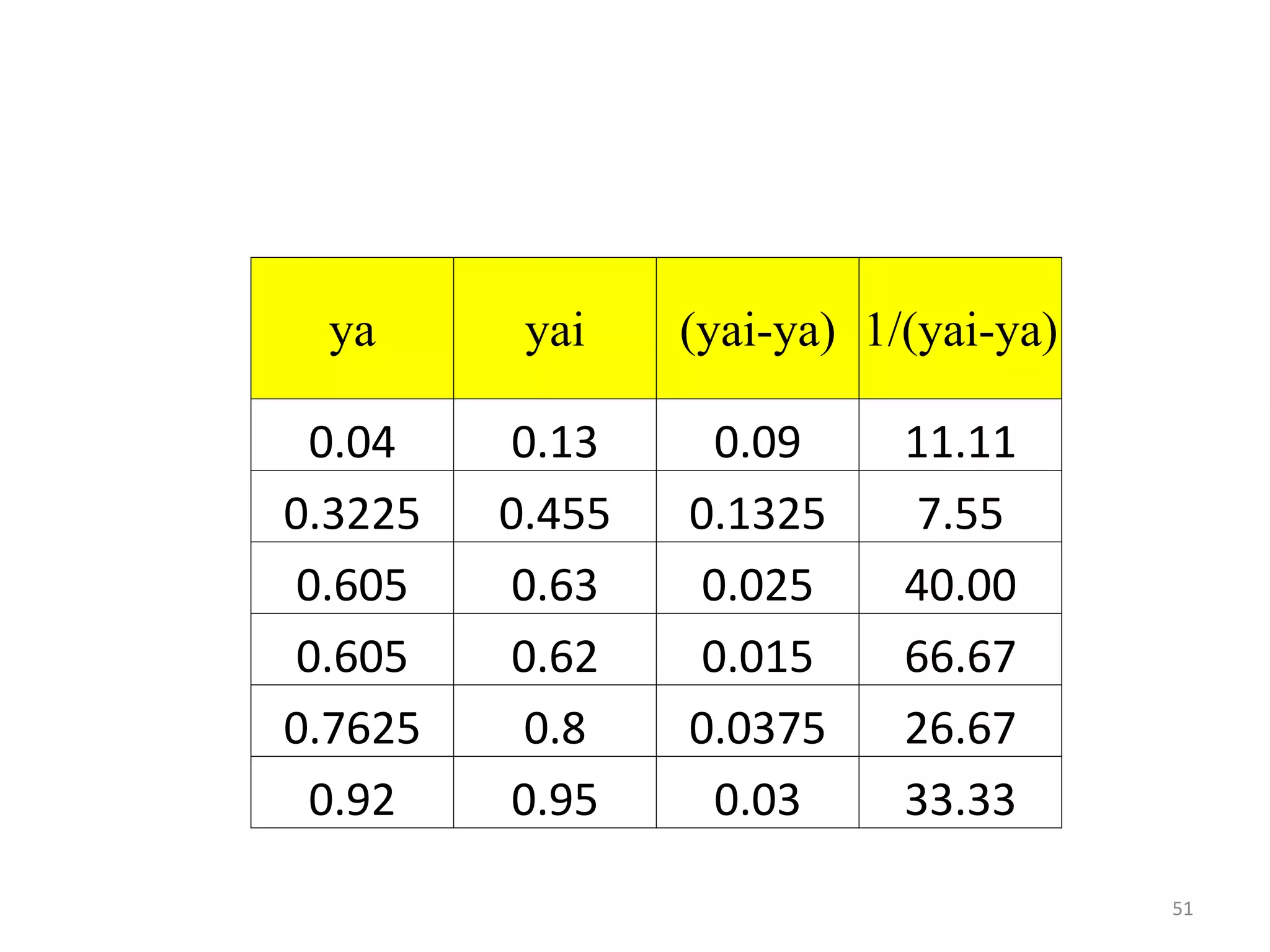
Packed columns are used for distillation, gas absorption, and liquid-liquid extraction. They have continuous gas-liquid contact through a packed bed, unlike plate columns which have stage-wise contact. Packed columns depend on good liquid and gas distribution, and have lower holdup but higher pressure drop than plate columns. This document provides details on packed column components, design procedures such as selecting packing and determining height, and examples of absorption and stripping processes in packed columns.