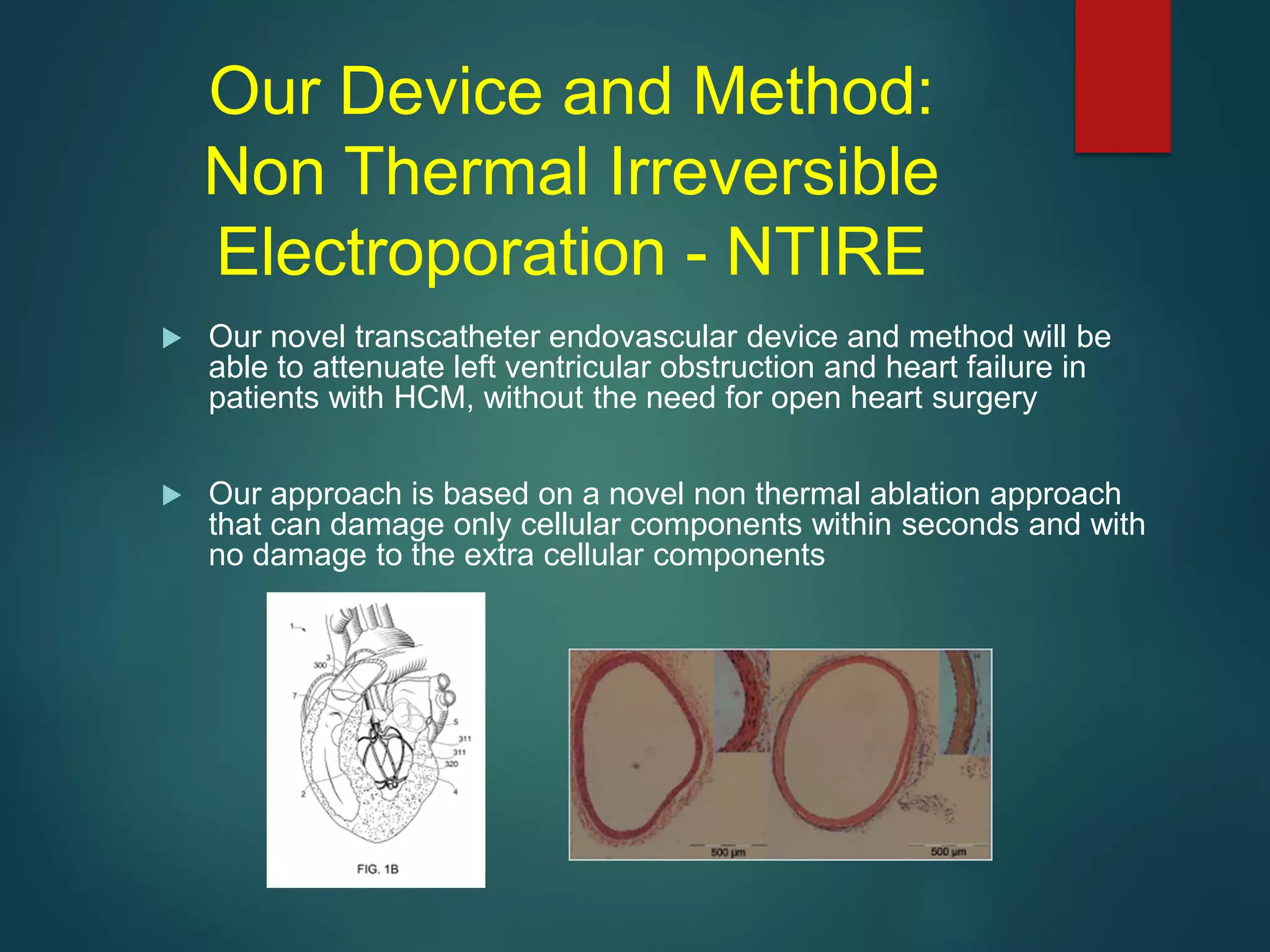
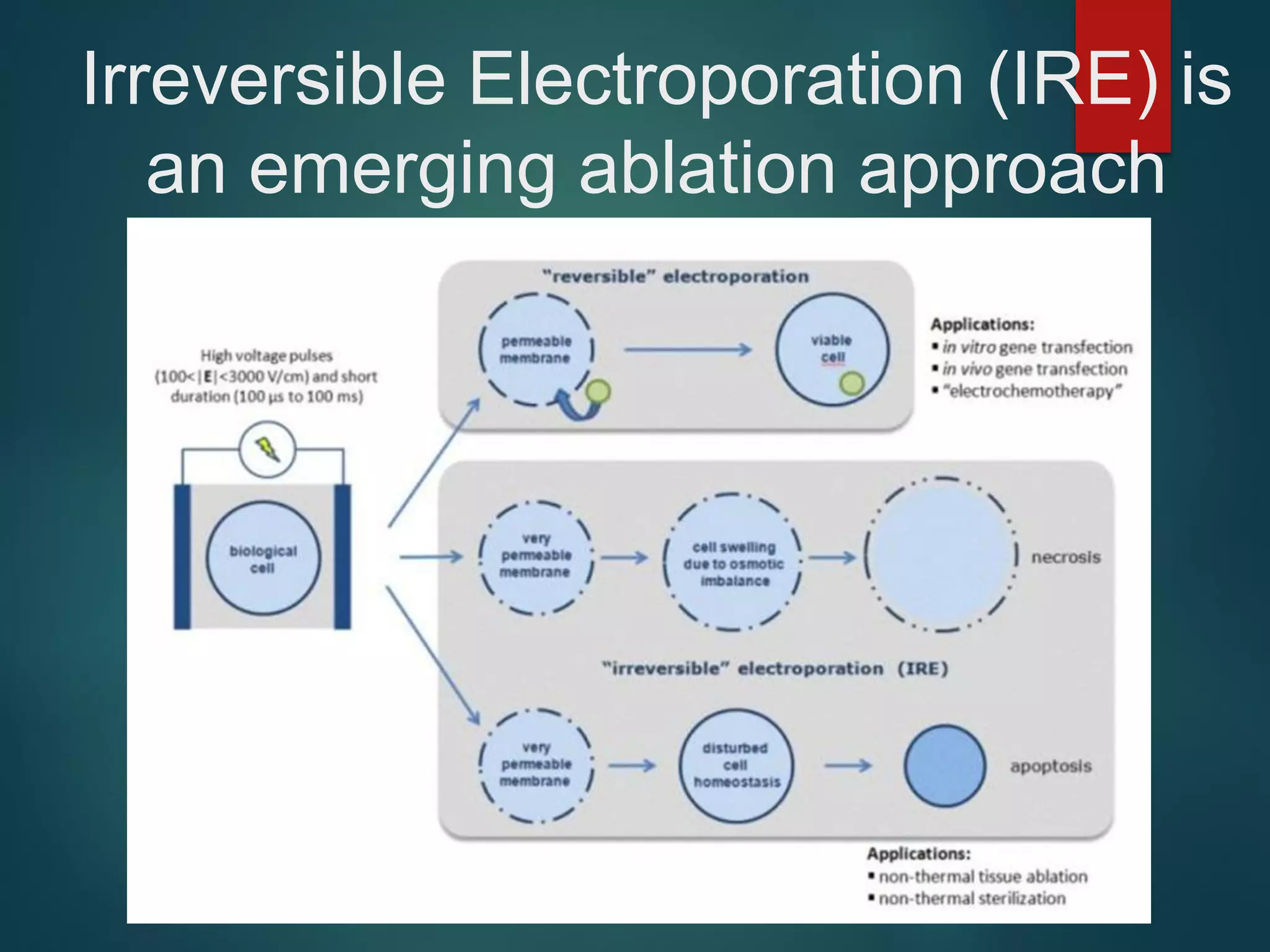
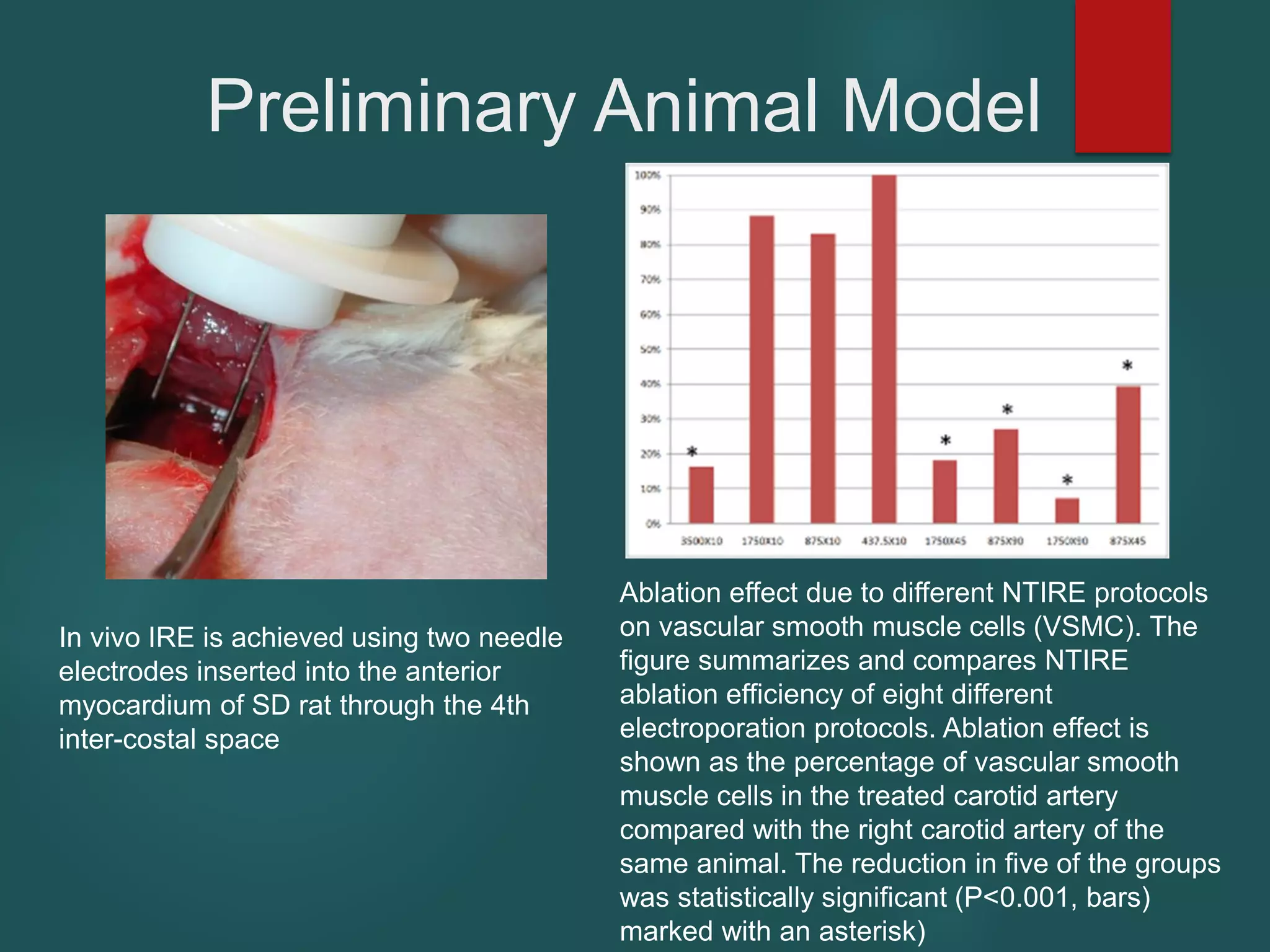
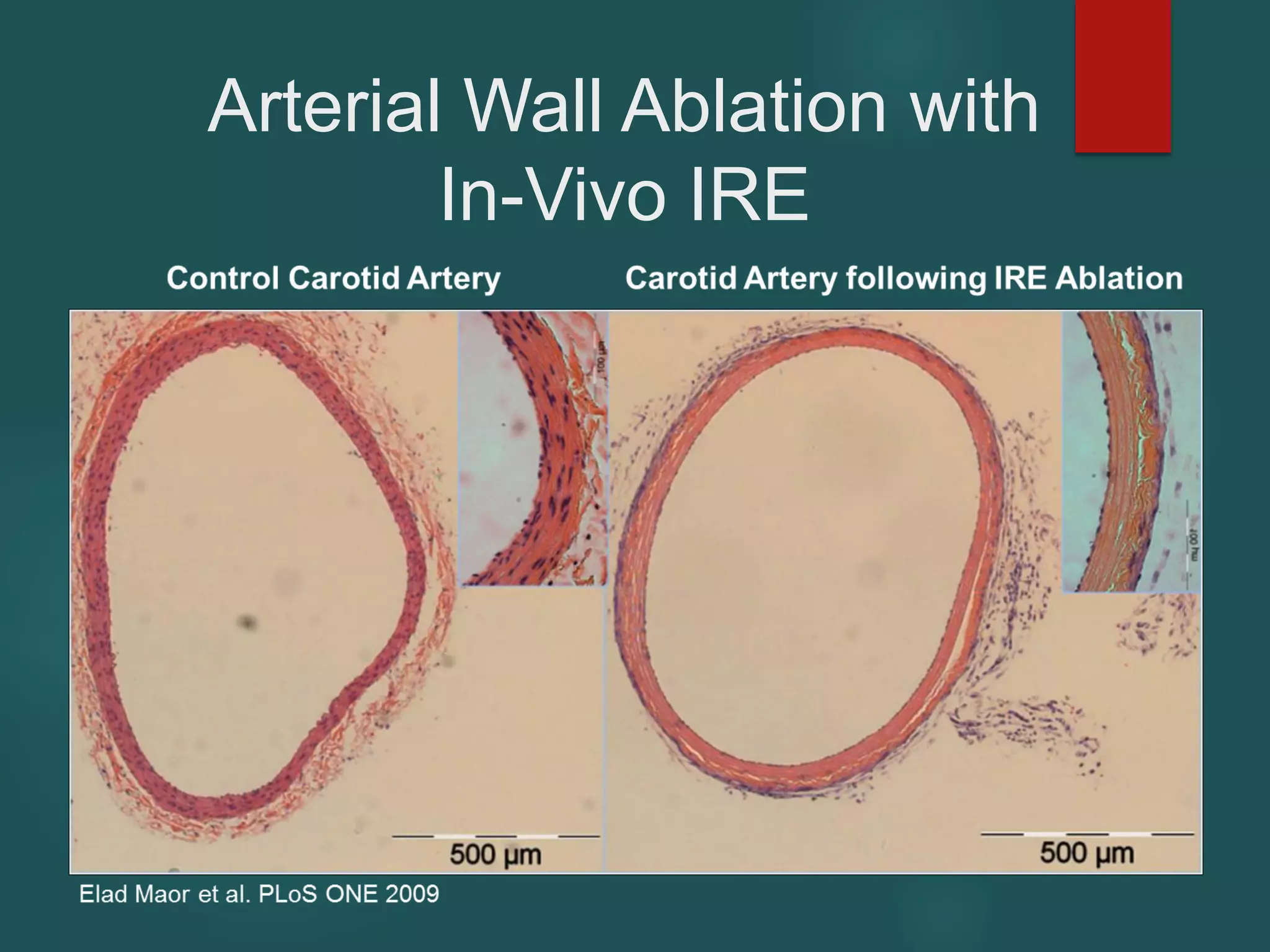
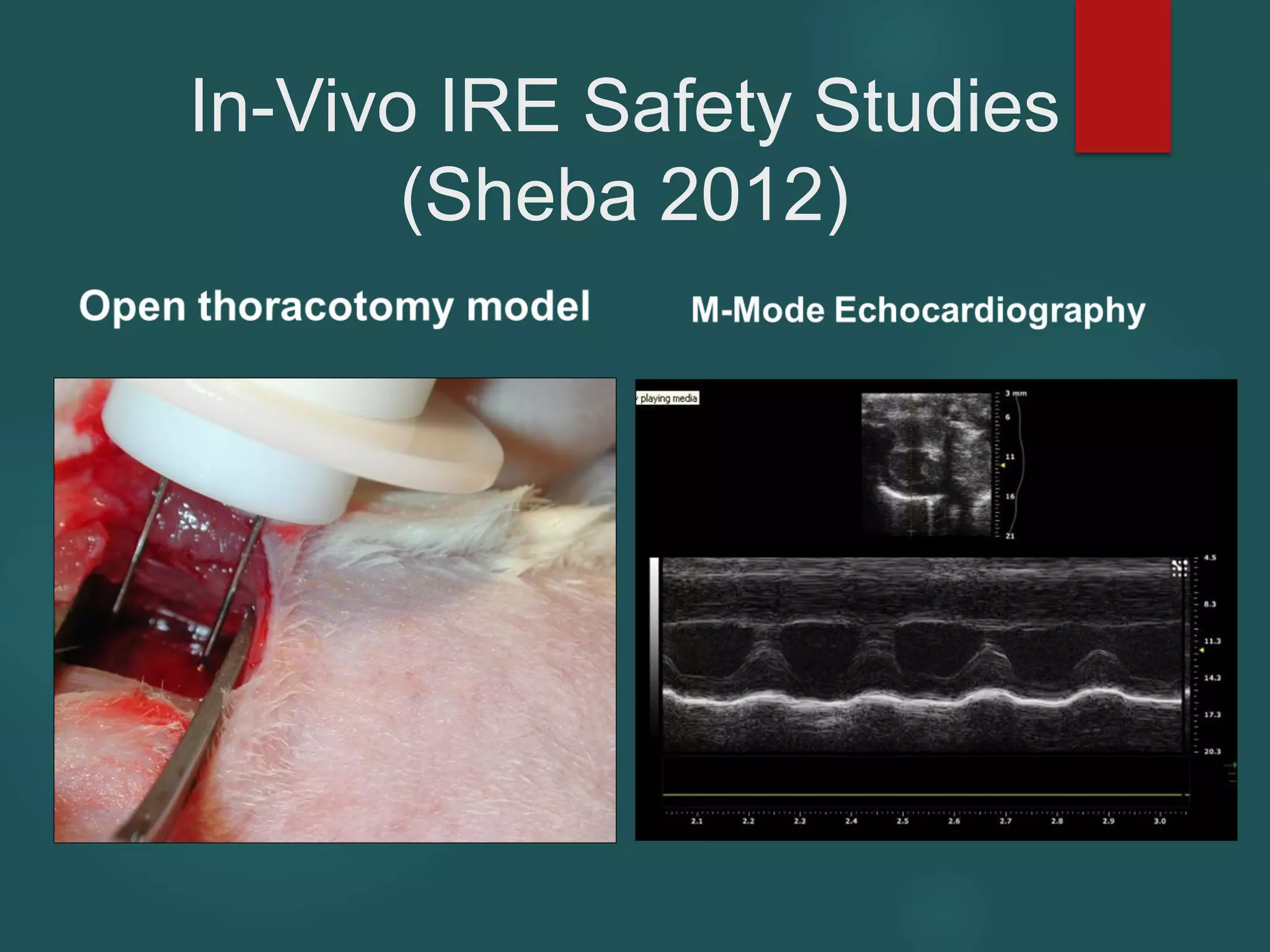
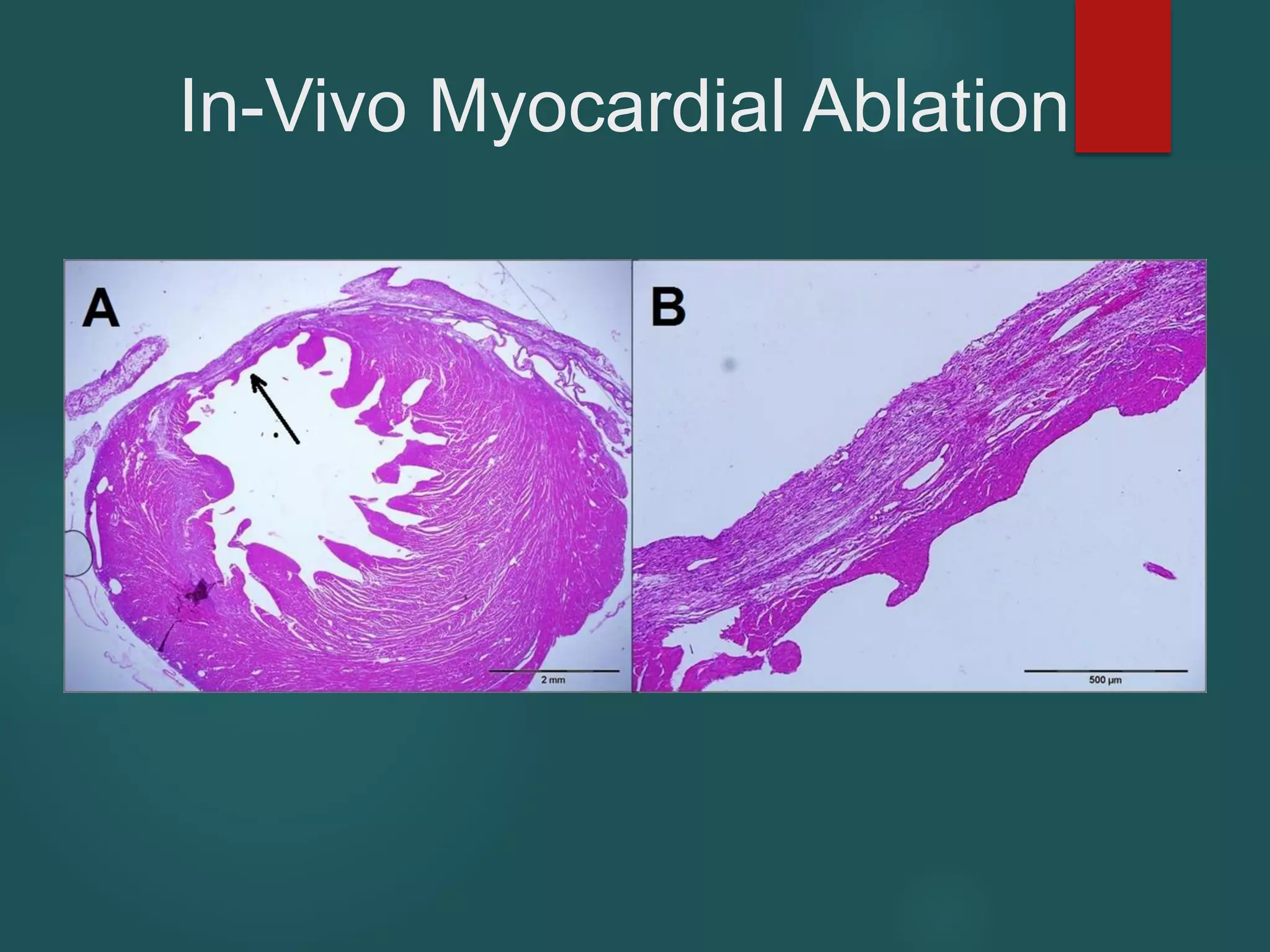
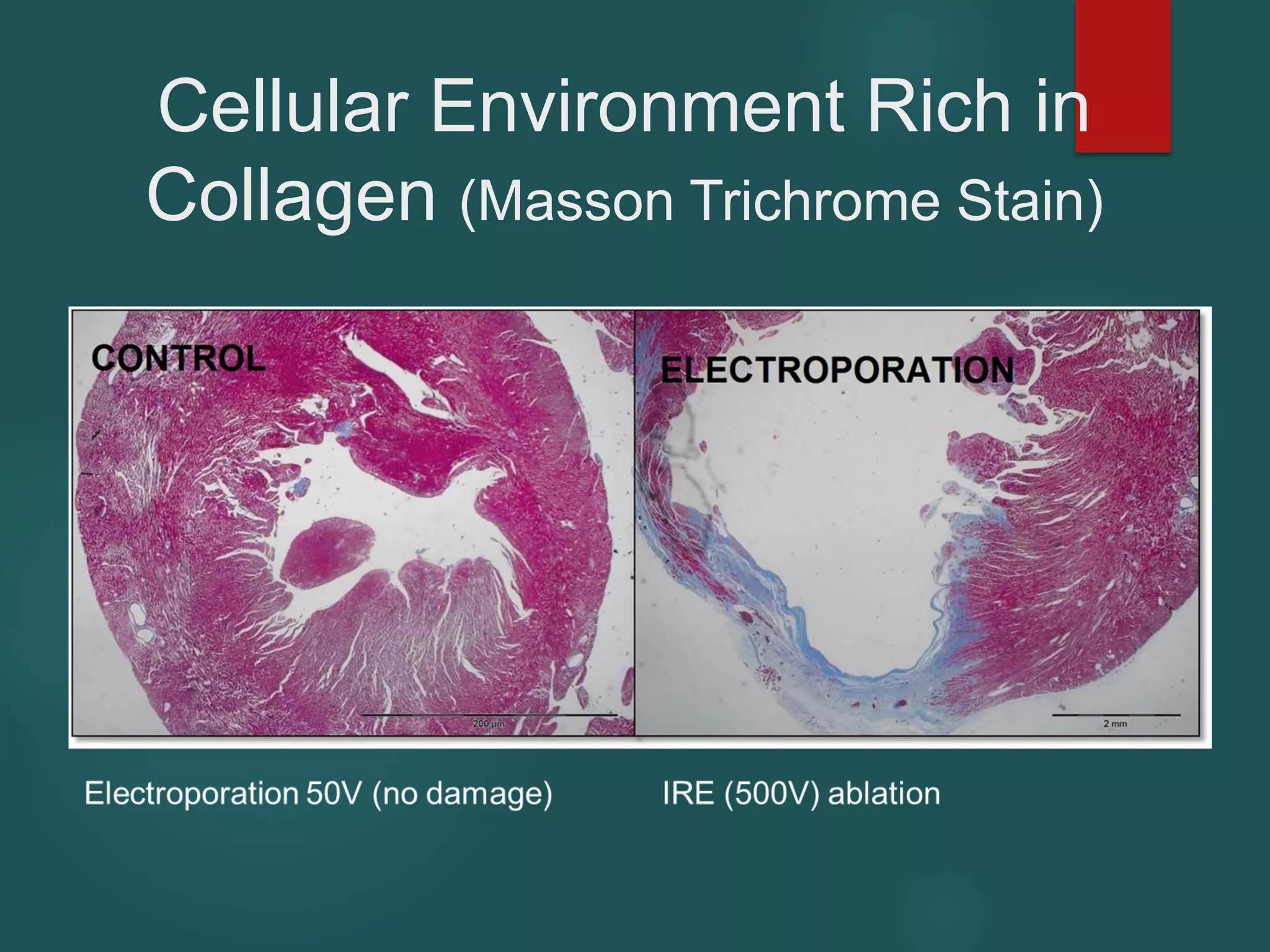
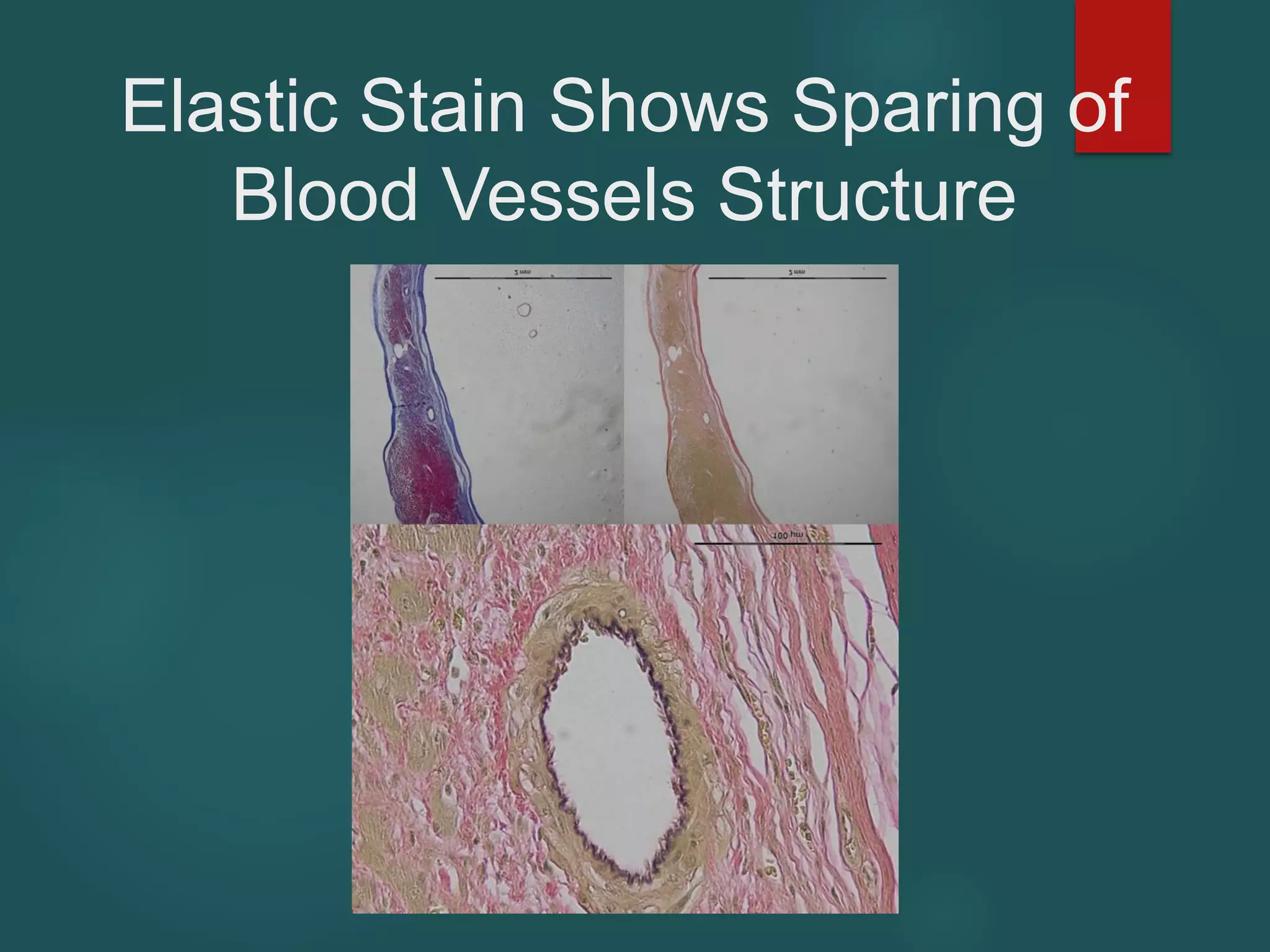
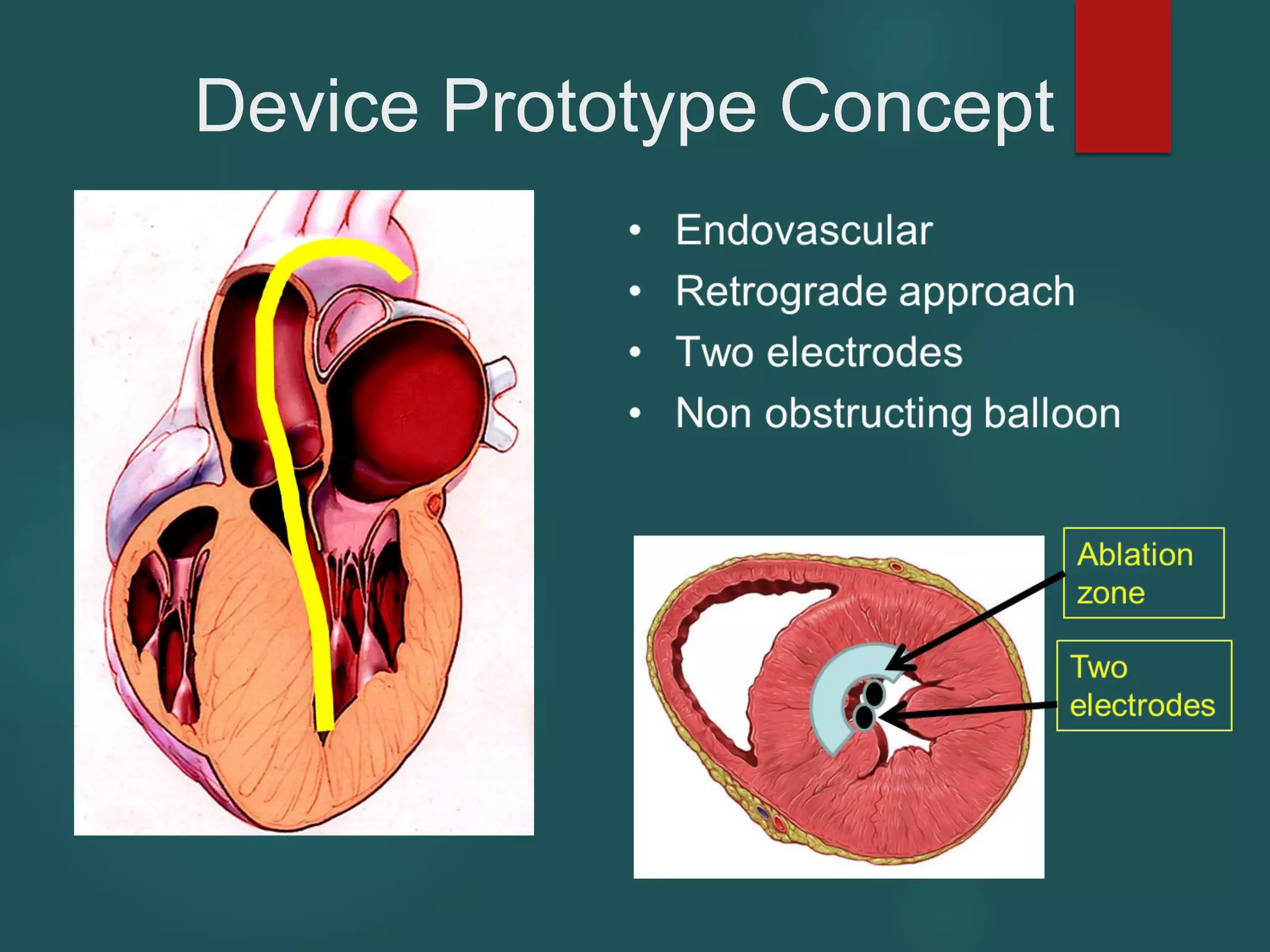
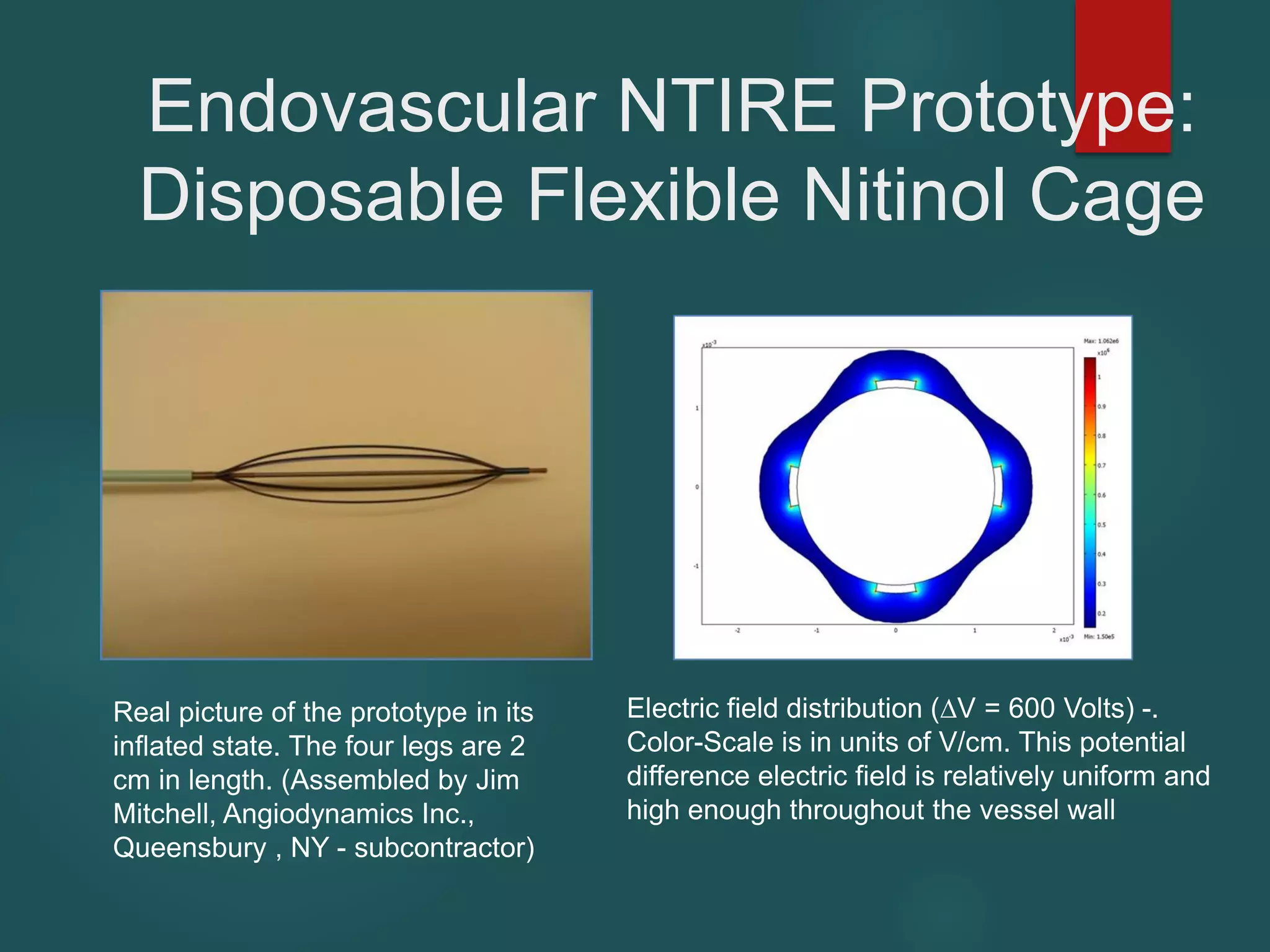
This document describes a novel transcatheter device to treat hypertrophic obstructive cardiomyopathy (HOCM) using non-thermal irreversible electroporation (NTIRE). HOCM is a common genetic heart condition that can cause sudden death. Current treatments have limitations. The proposed device uses NTIRE, which damages cell membranes without heat, to ablate thickened heart muscle in seconds via a minimally invasive approach. Preclinical studies show NTIRE safely and effectively ablates cardiac tissue. The team aims to develop an endovascular NTIRE device for HOCM and pursue FDA approval based on existing use in cancer. They expect the addressable market to be large with an opportunity to develop additional cardiac applications