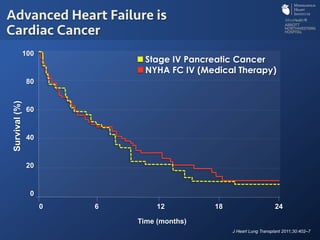
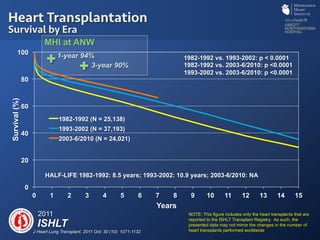
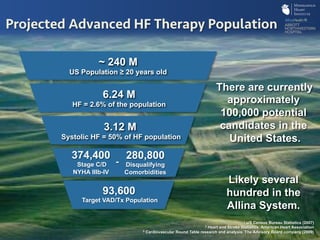
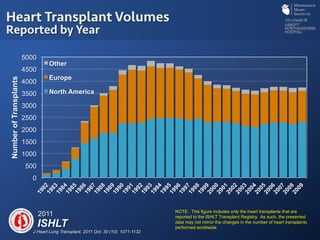
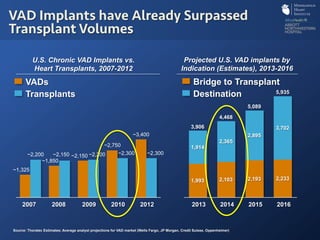
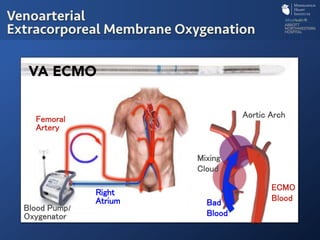
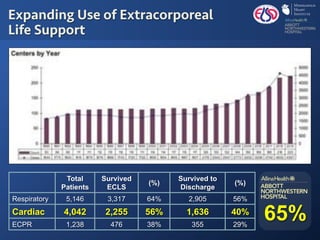
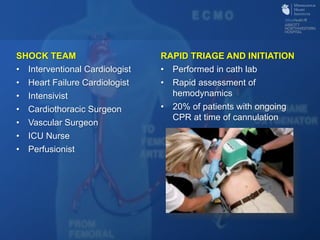
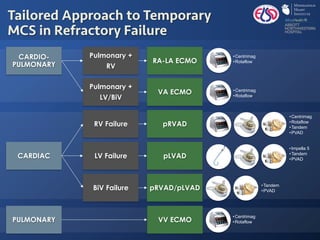
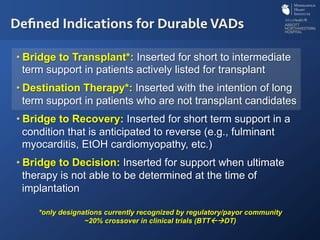
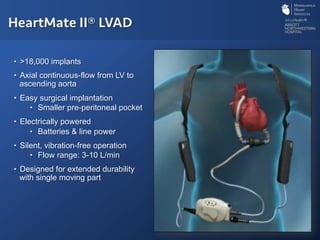
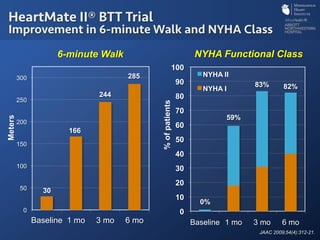
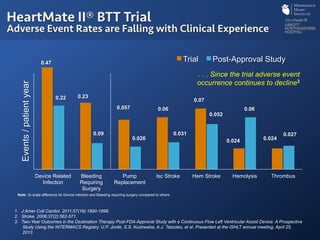
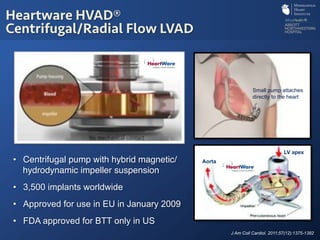
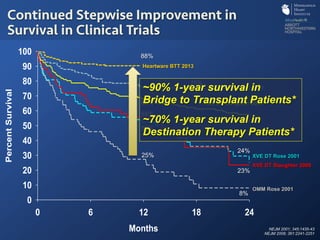
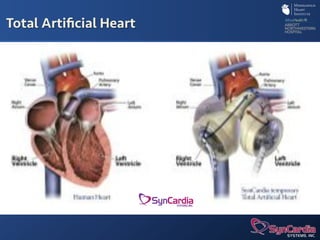
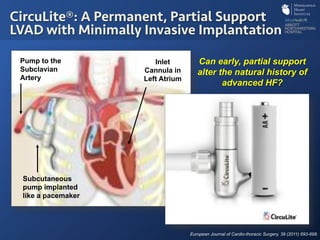
The document discusses advanced heart failure therapies, including cardiac transplantation and mechanical circulatory support, highlighting the increasing prevalence and survival rates of heart failure. It provides statistical data on heart transplants, mechanical support devices, and patient outcomes, underscoring trends in treatment effectiveness. The presentation includes references to various studies and treatments, aiming to inform on the evolving landscape of heart failure management.