Membranes pt. 2
•Download as PPT, PDF•
7 likes•1,560 views
Unit 4 pt. 2
Report
Share
Report
Share
Recommended
More Related Content
What's hot
What's hot (20)
Mechanism of vd(j) recombination and generation of antibody diversity

Mechanism of vd(j) recombination and generation of antibody diversity
Sorting and sorting and regulation of intracellular transport

Sorting and sorting and regulation of intracellular transport
Viewers also liked
Viewers also liked (20)
Integral and peripheral membrane proteins medical images for power point

Integral and peripheral membrane proteins medical images for power point
Membrane based classification of signalling pathways

Membrane based classification of signalling pathways
Drug Dependence & Abuse - Presentation by Akshay Anand

Drug Dependence & Abuse - Presentation by Akshay Anand
G protein coupled receptor and pharmacotherapeutics

G protein coupled receptor and pharmacotherapeutics
Similar to Membranes pt. 2
Similar to Membranes pt. 2 (20)
Molecular interaction, Regulation and Signalling receptors and vesicles

Molecular interaction, Regulation and Signalling receptors and vesicles
11.15 (dr. husun bano) cell signalling mechanisms 1st & 2nd

11.15 (dr. husun bano) cell signalling mechanisms 1st & 2nd
11.16 (dr. surriya sheikh) cell signalling 1 1 neurotransmitter

11.16 (dr. surriya sheikh) cell signalling 1 1 neurotransmitter
Cell signaling(signaling through g protien coupled receptors,signal transduct...

Cell signaling(signaling through g protien coupled receptors,signal transduct...
CELL SIGNALING AND PATHWAYS INVOLVED IN CELL SIGNALING AND SOLID TUMORS.

CELL SIGNALING AND PATHWAYS INVOLVED IN CELL SIGNALING AND SOLID TUMORS.
Cell communications in Cell Biology Becker's Textbook

Cell communications in Cell Biology Becker's Textbook
More from Jolie Yu
More from Jolie Yu (20)
Recently uploaded
APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across SectorsAssociation for Project Management
Recently uploaded (20)
Kisan Call Centre - To harness potential of ICT in Agriculture by answer farm...

Kisan Call Centre - To harness potential of ICT in Agriculture by answer farm...
JAPAN: ORGANISATION OF PMDA, PHARMACEUTICAL LAWS & REGULATIONS, TYPES OF REGI...

JAPAN: ORGANISATION OF PMDA, PHARMACEUTICAL LAWS & REGULATIONS, TYPES OF REGI...
Russian Call Girls in Andheri Airport Mumbai WhatsApp 9167673311 💞 Full Nigh...

Russian Call Girls in Andheri Airport Mumbai WhatsApp 9167673311 💞 Full Nigh...
Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...

Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...
Web & Social Media Analytics Previous Year Question Paper.pdf

Web & Social Media Analytics Previous Year Question Paper.pdf
APM Welcome, APM North West Network Conference, Synergies Across Sectors

APM Welcome, APM North West Network Conference, Synergies Across Sectors
Interactive Powerpoint_How to Master effective communication

Interactive Powerpoint_How to Master effective communication
Measures of Central Tendency: Mean, Median and Mode

Measures of Central Tendency: Mean, Median and Mode
Separation of Lanthanides/ Lanthanides and Actinides

Separation of Lanthanides/ Lanthanides and Actinides
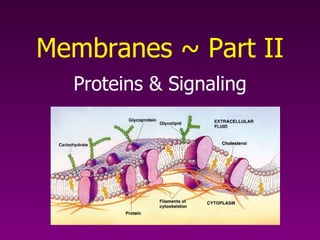
Membranes pt. 2
- 1. Proteins & Signaling Membranes ~ Part II
- 4. Mechanisms of Cell Communication
- 9. Ligand Gated Ion Channel
- 18. An Overview of Cell Signaling
- 26. Tyrosine Kinase Receptor Dimers
- 30. Exchange of Yeast Mating Factors
- 43. Cyclic AMP
- 45. cAMP Second Messenger System