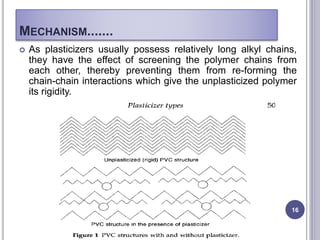
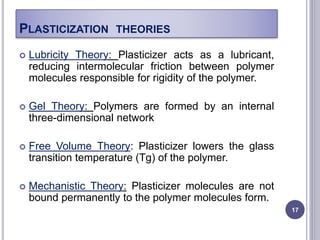
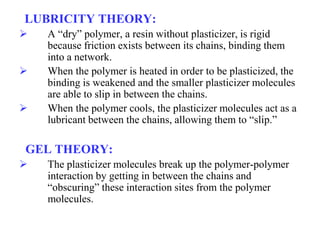
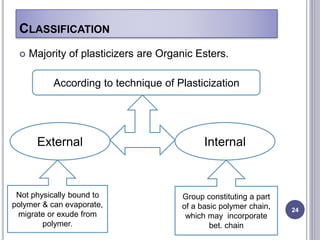
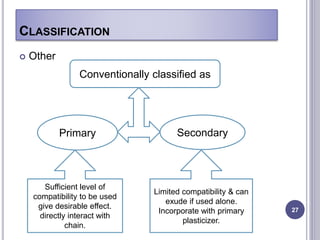
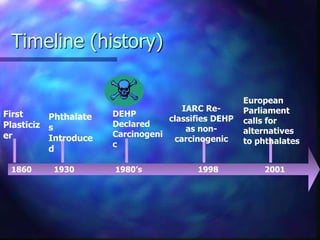
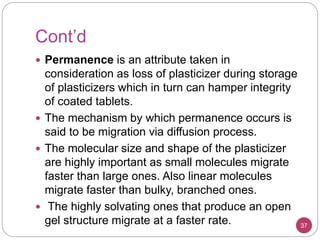
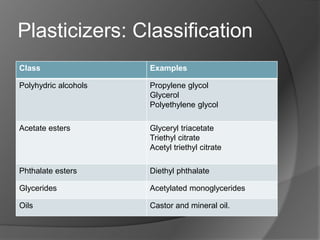
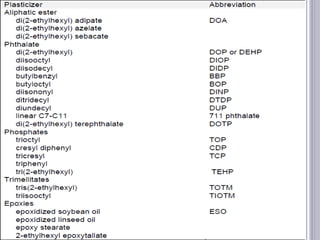
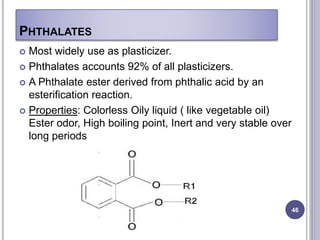
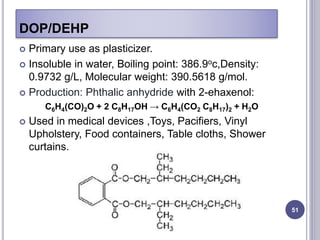
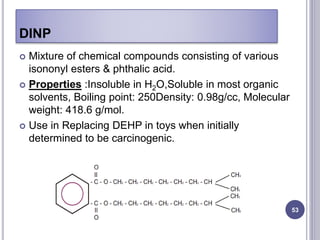
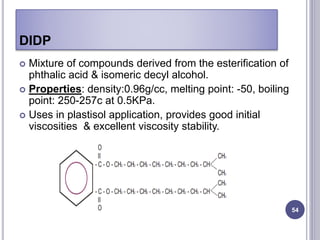
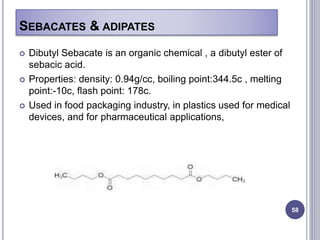
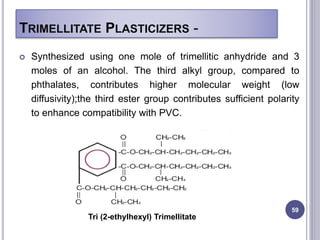
Plasticizers are substances added to polymers to make them softer and more flexible. They work by inserting themselves between polymer chains and reducing intermolecular forces and friction. This lowers the glass transition temperature of the polymer. Common plasticizers are esters such as phthalates, adipates, and trimellitates. They are added to things like plastics, cosmetics, and pharmaceutical films to modify properties.