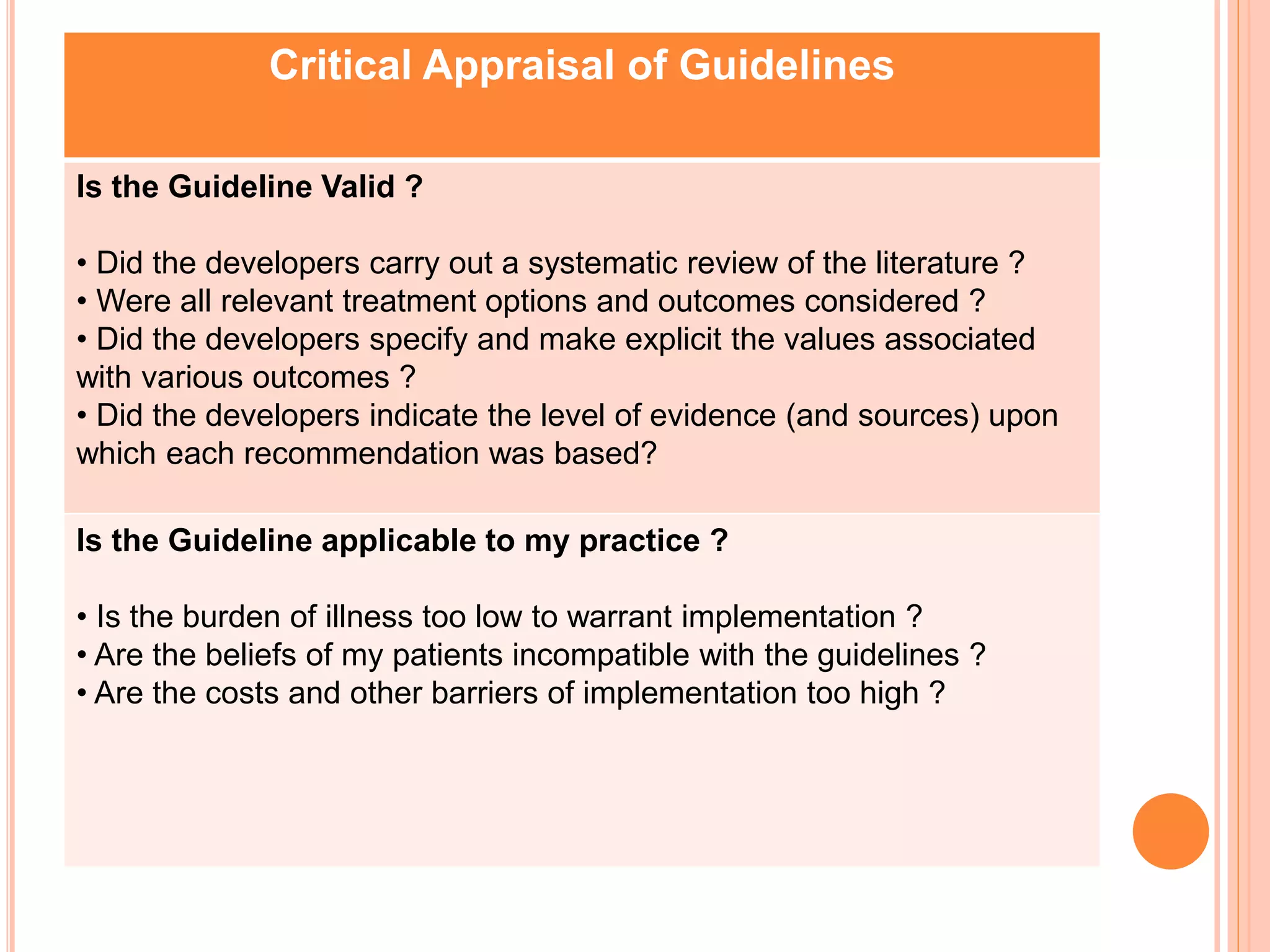
This document discusses clinical practice guidelines and their role in evidence-based practice. It provides definitions of clinical practice guidelines and discusses their increased use due to concerns over variability in care, costs, quality and liability. It notes guidelines can differ in comprehensiveness, format, review frequency and ease of use. While guidelines are distinct from evidence-based practice, high quality evidence-based guidelines including a systematic literature review can provide useful guidance. The document lists sources of guidelines and outlines a six-step process for developing evidence-based practice guidelines, including identifying topics, convening experts, systematically reviewing evidence, translating evidence into recommendations, using outside reviewers, and periodic updates. It also discusses critically appraising guidelines for validity and applicability.