Embed presentation
Download to read offline

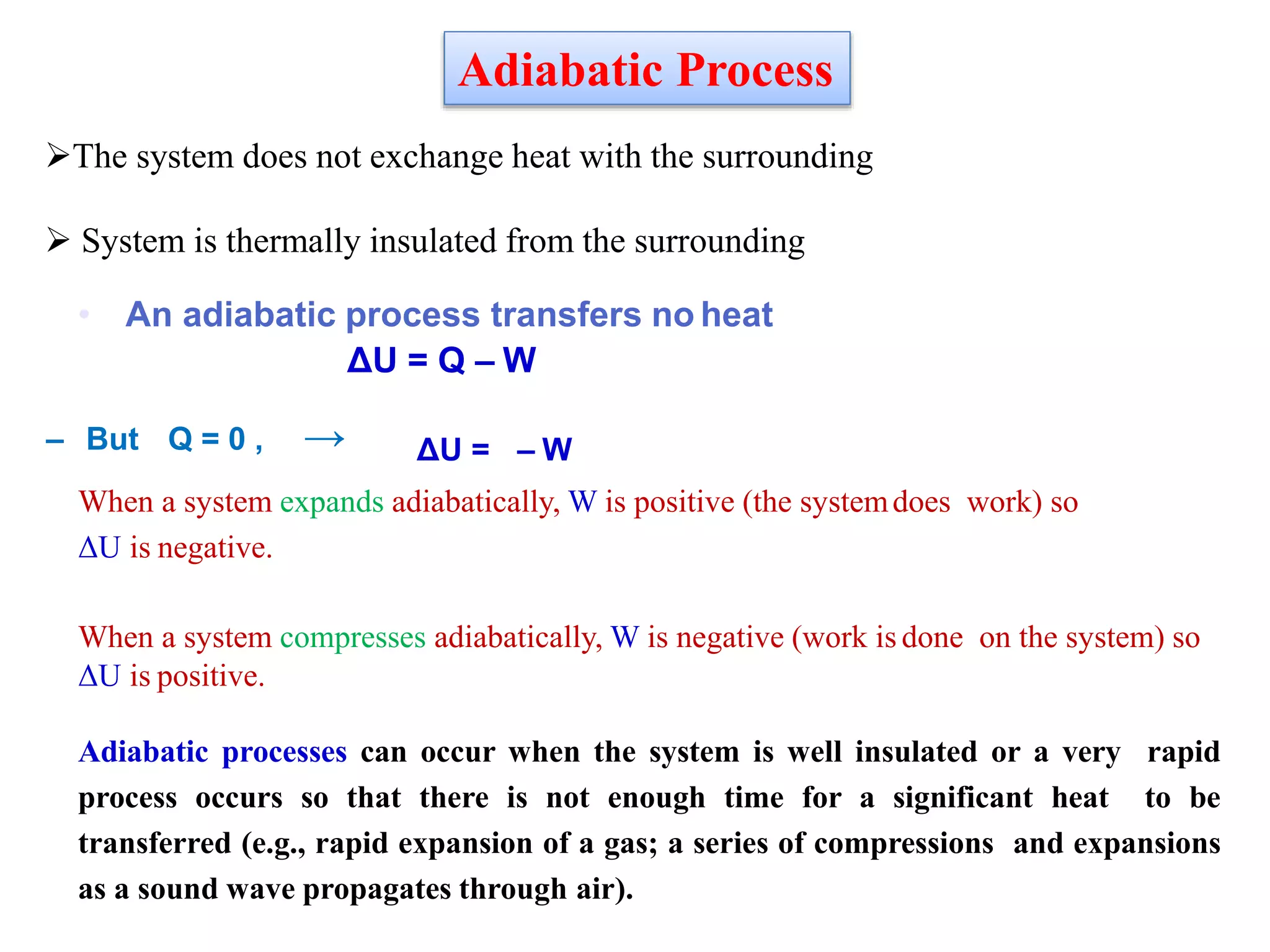


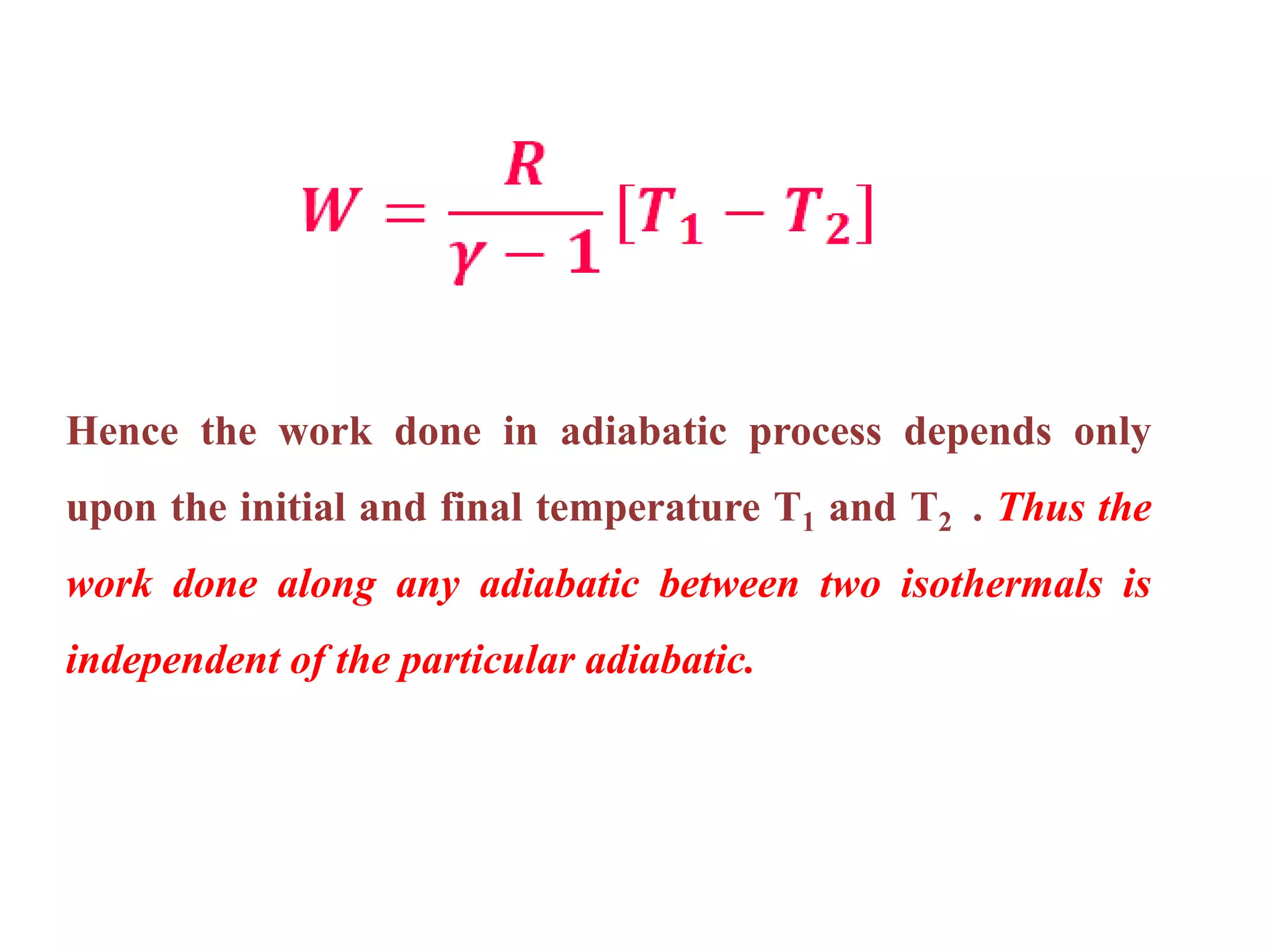


This document discusses work done during an adiabatic process. It defines an adiabatic process as one where no heat is exchanged between a system and its surroundings. The document states that for an adiabatic expansion, work is done by the system so its internal energy decreases, while for an adiabatic compression, work is done on the system so its internal energy increases. It also explains that the work done in an adiabatic process depends only on the initial and final temperatures of the system.





