Embed presentation
Download to read offline

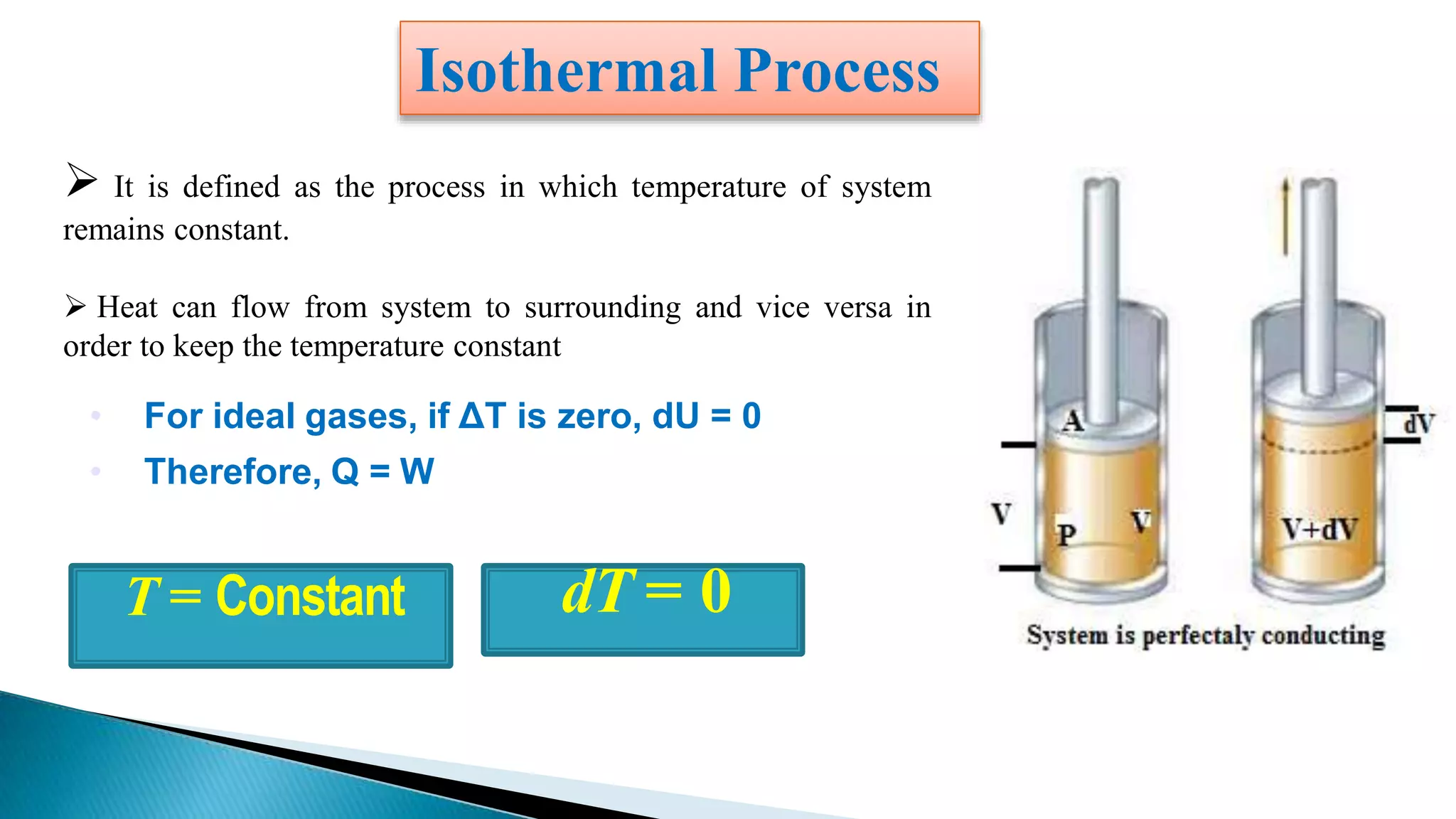
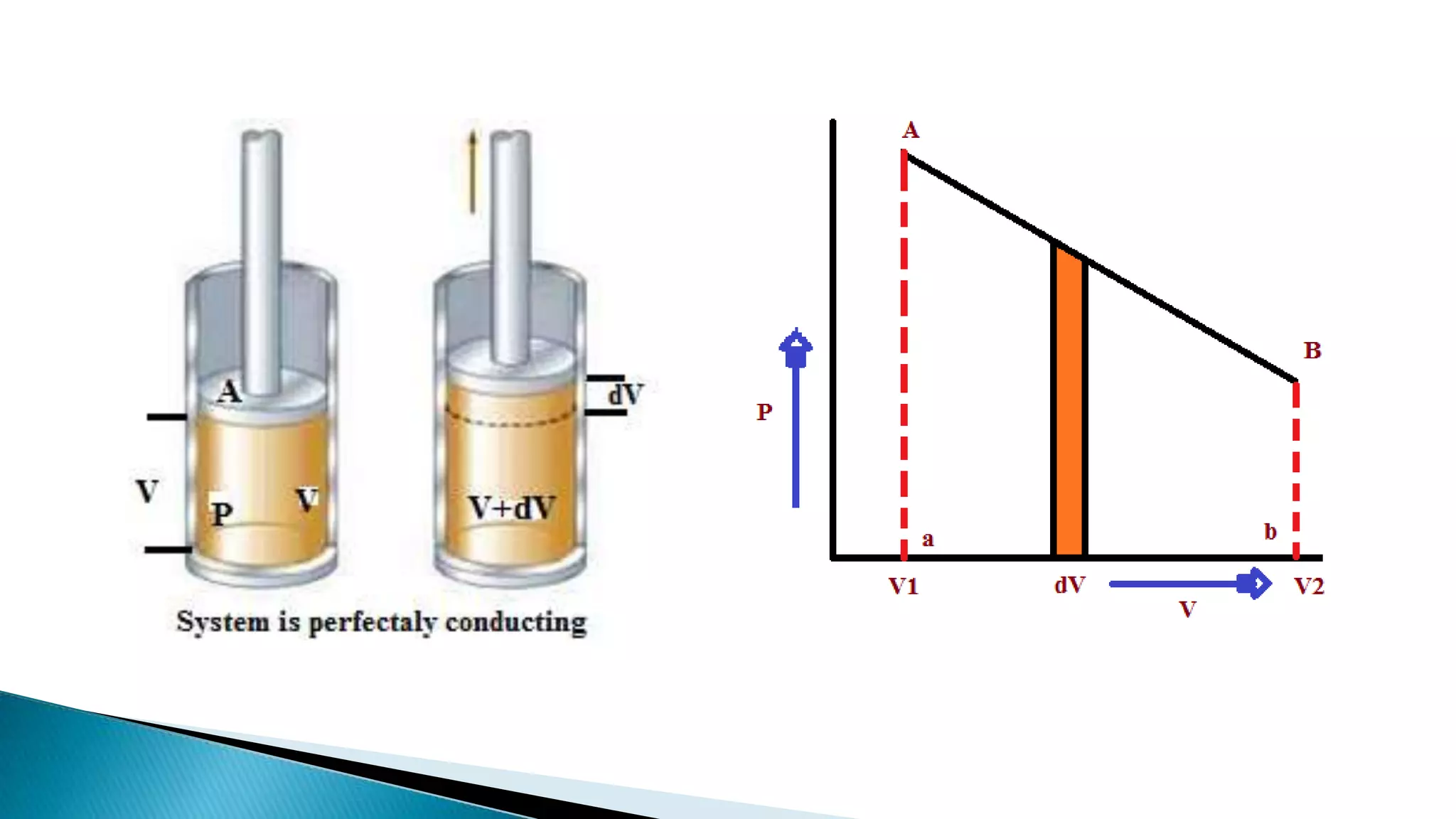
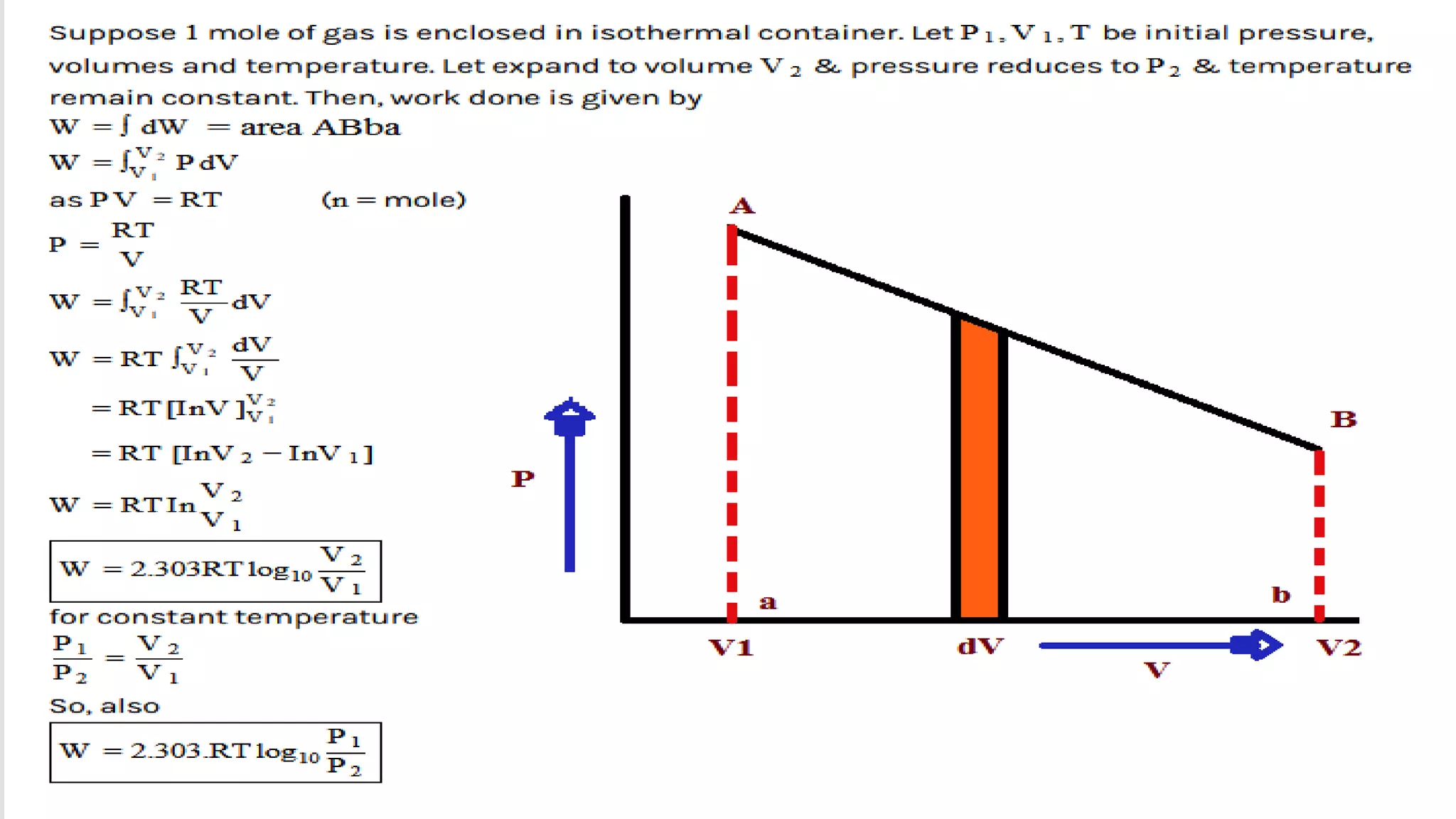


This document discusses work done during an isothermal process. An isothermal process is defined as one where the temperature of the system remains constant. Heat can flow from the system to the surroundings and vice versa in order to keep the temperature constant. For ideal gases, if the change in temperature is zero, the change in internal energy is also zero, so the heat transferred equals the work done.




