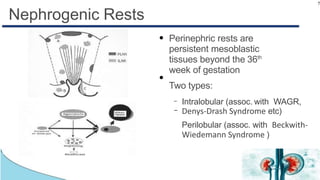
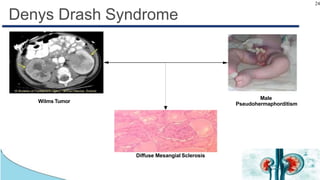
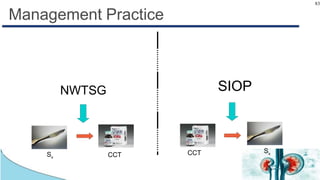
Wilms tumor, also known as nephroblastoma, is the most common renal tumor of childhood. It has an annual incidence of 7.6 cases per million children under 15 years old. Treatment involves surgery to remove the tumor along with chemotherapy and sometimes radiation therapy in a multimodal approach. The goal is to remove the tumor bulk surgically while using chemotherapy to eliminate any micrometastases in order to cure the cancer. Protocols vary depending on factors like age, tumor stage and histology, but generally include either surgery followed by chemotherapy or neoadjuvant chemotherapy before surgery, with excellent long-term survival rates with modern therapies.