- Weathering is the breakdown of rocks and minerals due to contact with water, air, and organisms. It occurs on site as opposed to erosion, which involves transport.
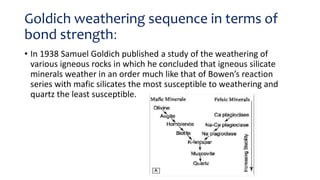
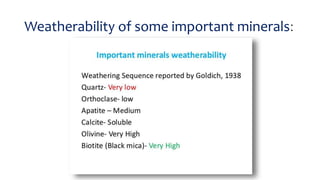
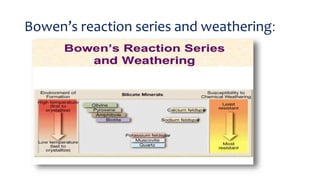
- Goldich's weathering sequence predicts that minerals formed under higher temperatures and pressures are less stable at the earth's surface than low-temperature minerals. The sequence follows Bowen's reaction series, with early-crystallizing minerals weathering first.
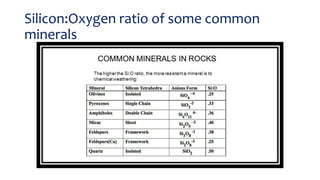
- The rate of weathering depends on factors like pH, temperature, surface area, competing ions, and fluid flow rates. Minerals with higher silicon-to-oxygen ratios are more resistant to weathering.







![Weathering mechanism:
• The general dissolution reaction of silicates in inorganic aqueous
system involves multi step process of initial rapid exchange of cations
for protons at the mineral surface followed by a slow rate
determining hydrolysis. And subsequent detachment of silica and
alumina species from the remaining framework (Aagaard and
Helgeson, 1982)
• Destruction of framework bonds are known to occur through
decomposition of these surface complexes when they are in activated
state.
• Therefore the overall dissolution rate can be expressed as
Rate=k[s]](https://image.slidesharecdn.com/weatheringsequence-211013033845/85/Weathering-sequence-9-320.jpg)