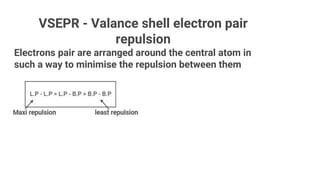
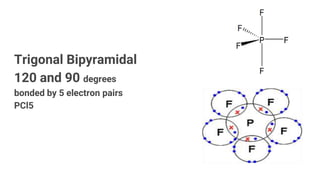
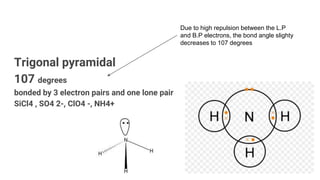
VSEPR theory uses the valence shell electron pair repulsion model to predict the shapes of molecules based on electron pairs. It states that electron pairs will adopt an arrangement that minimizes repulsion. Examples given are trigonal planar for BF3, linear for CO2, tetrahedral for CH4, and octahedral for SF6. The theory explains that molecular geometry is determined by the number of electron pairs (both bonding pairs and lone pairs) around the central atom and how the pairs repel one another to maximize distance.