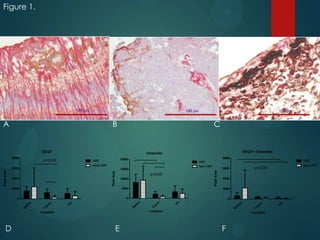
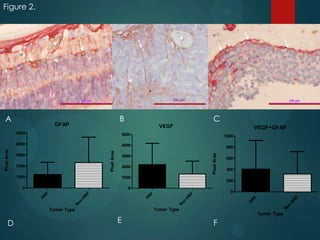
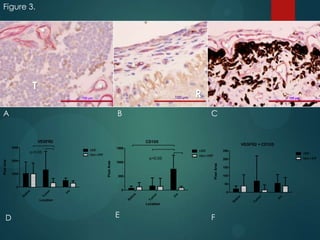
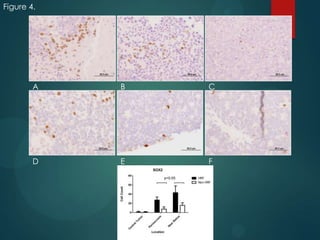
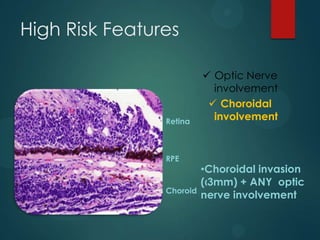
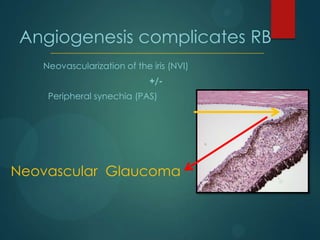
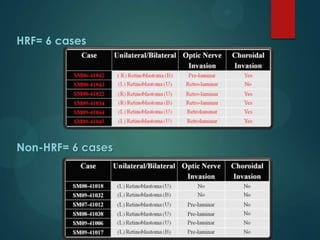
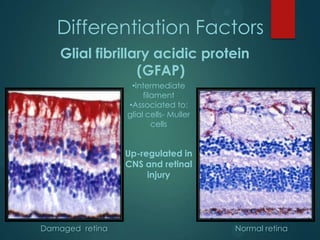
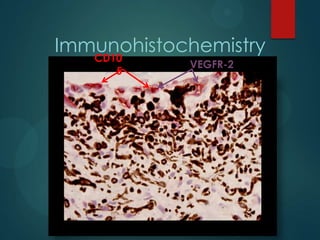
This study analyzed the expression of angiogenic factors in retinoblastoma tumors with high-risk features (HRF) compared to those without. The results showed that HRF tumors expressed significantly higher levels of VEGF and VEGFR2 than non-HRF tumors. Staining indicated the VEGF was secreted by tumor cells. Neovascularization was also higher in the iris of HRF tumors. Expression of VEGF correlated with the stem cell marker SOX2, suggesting HRF tumors are more stem-like. The conclusions were that HRF tumors may regulate invasiveness through a VEGF feedback loop, and anti-VEGF therapy may be a promising treatment for retinoblastoma due to these findings.






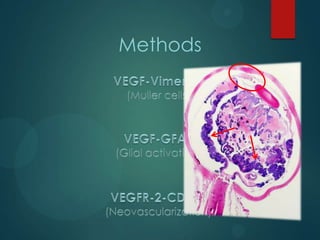
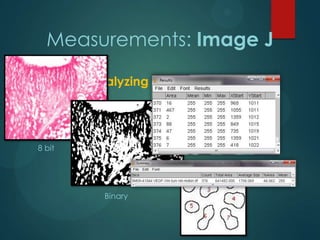
![Measurements: Image J
Color deconvolution
Stain separation using Ruifrok and Johnston's method1
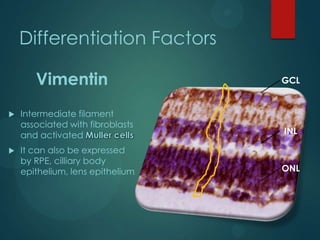
Vimentin
VEGF
[1] Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal
Quant Cytol Histol 23: 291-299, 2001](https://image.slidesharecdn.com/vegfexpressioninhrf-131111154300-phpapp02/85/Vegf-expression-in-hrf-17-320.jpg)