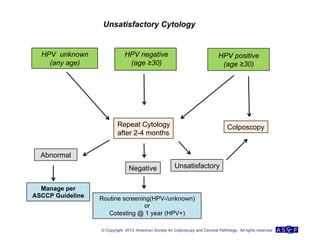
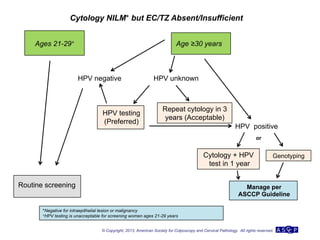
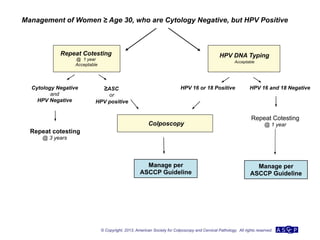
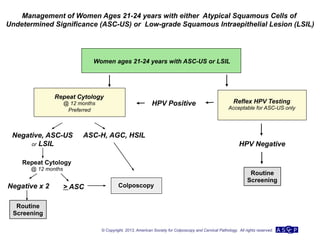
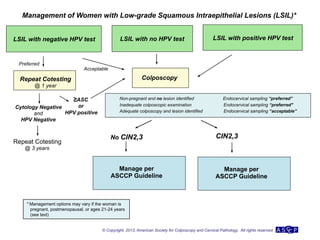
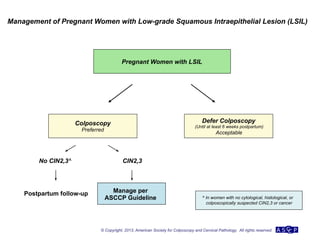
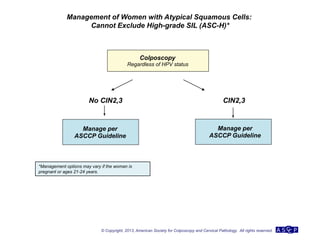
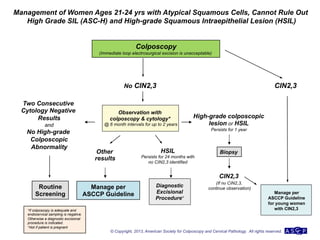
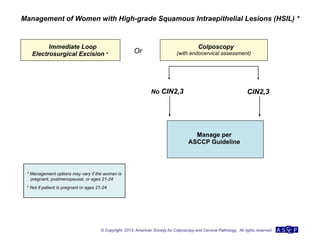
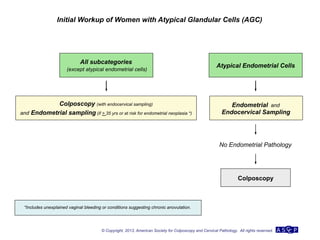
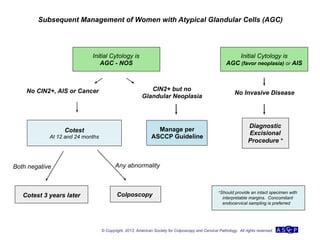
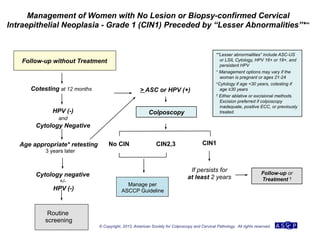
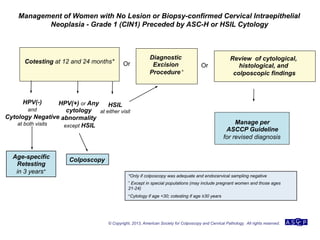
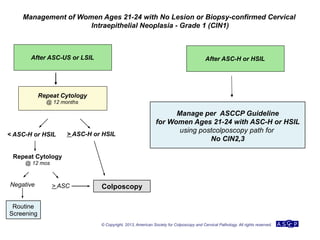
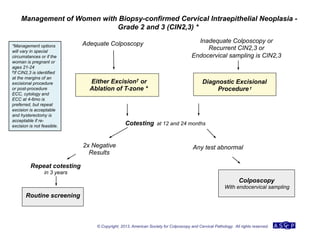
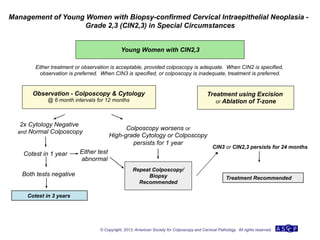
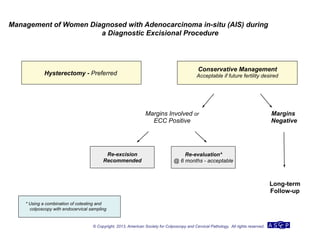
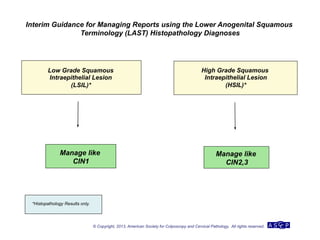
The document provides updated guidelines from the American Society for Colposcopy and Cervical Pathology (ASCCP) for managing abnormal cervical cancer screening tests and cervical precancer. Key changes include new screening guidelines, management of HPV-positive women under 30, and updated recommendations for evaluating cytological abnormalities, cervical precancer, and histopathological diagnoses. The guidelines are based on evidence from over 1 million women and aim to help clinicians better manage cervical cancer screening and precancer treatment.