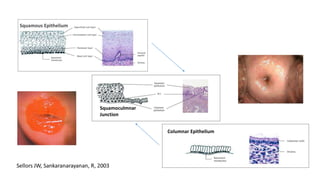
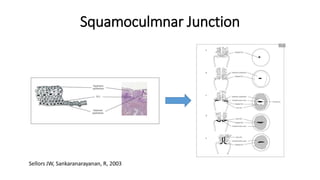
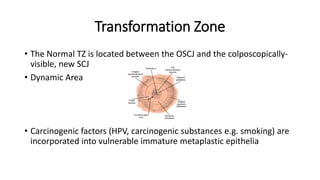
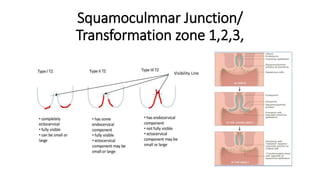
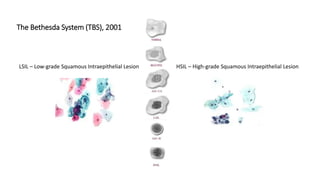
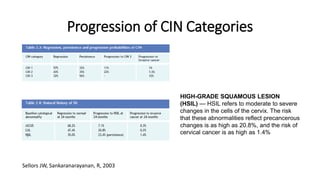
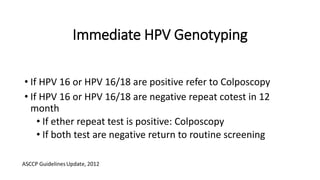
The document discusses the prevalence and screening methods for cervical cancer, highlighting the importance of understanding various types of abnormal Pap test results and their implications. It outlines guidelines for cervical cancer screening based on age and risk factors, emphasizing co-testing and follow-up procedures for abnormal results. Additionally, the document stresses the need for training healthcare providers to improve maternal health and gynecology services globally.