Embed presentation
Downloaded 30 times






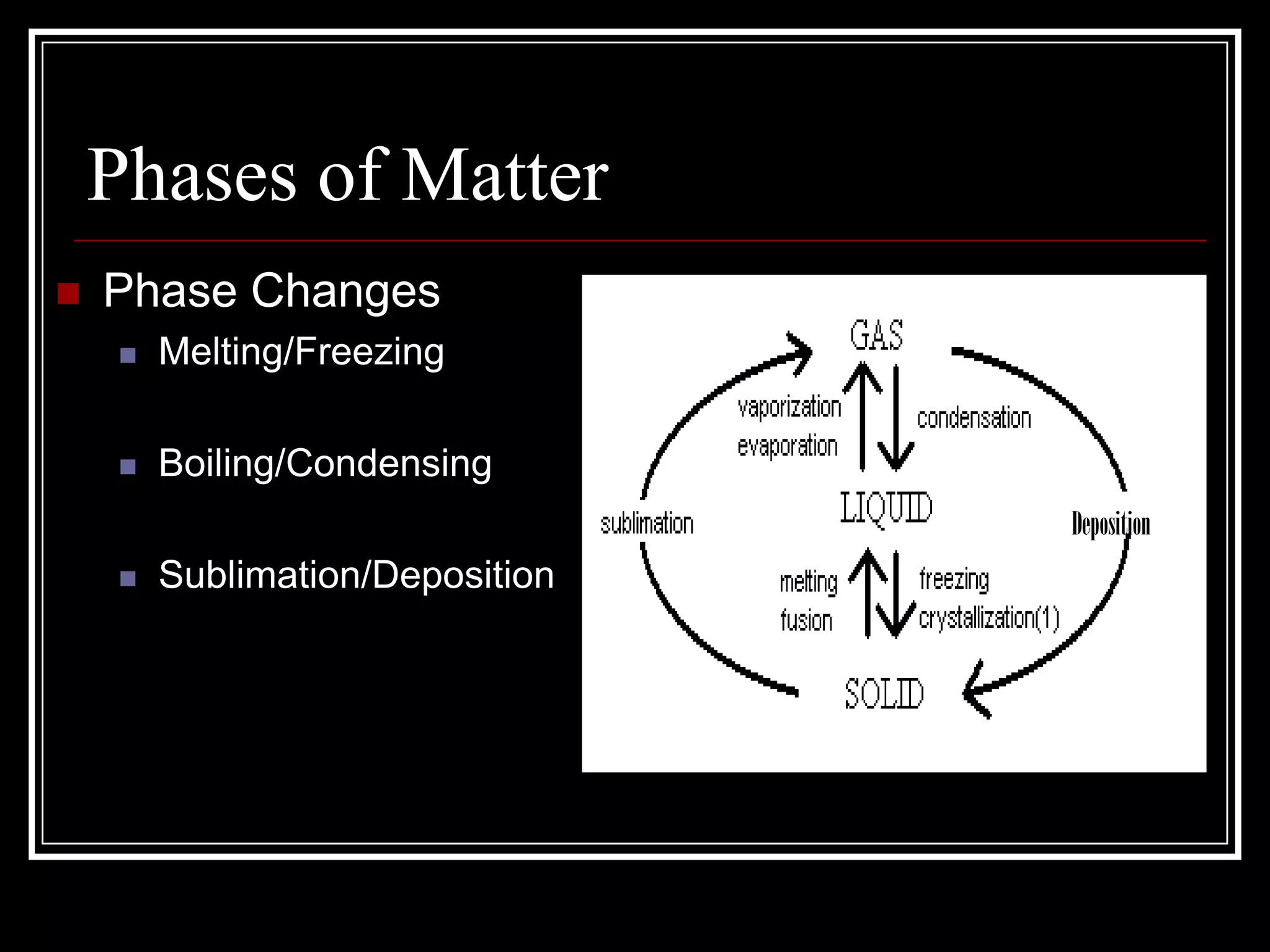

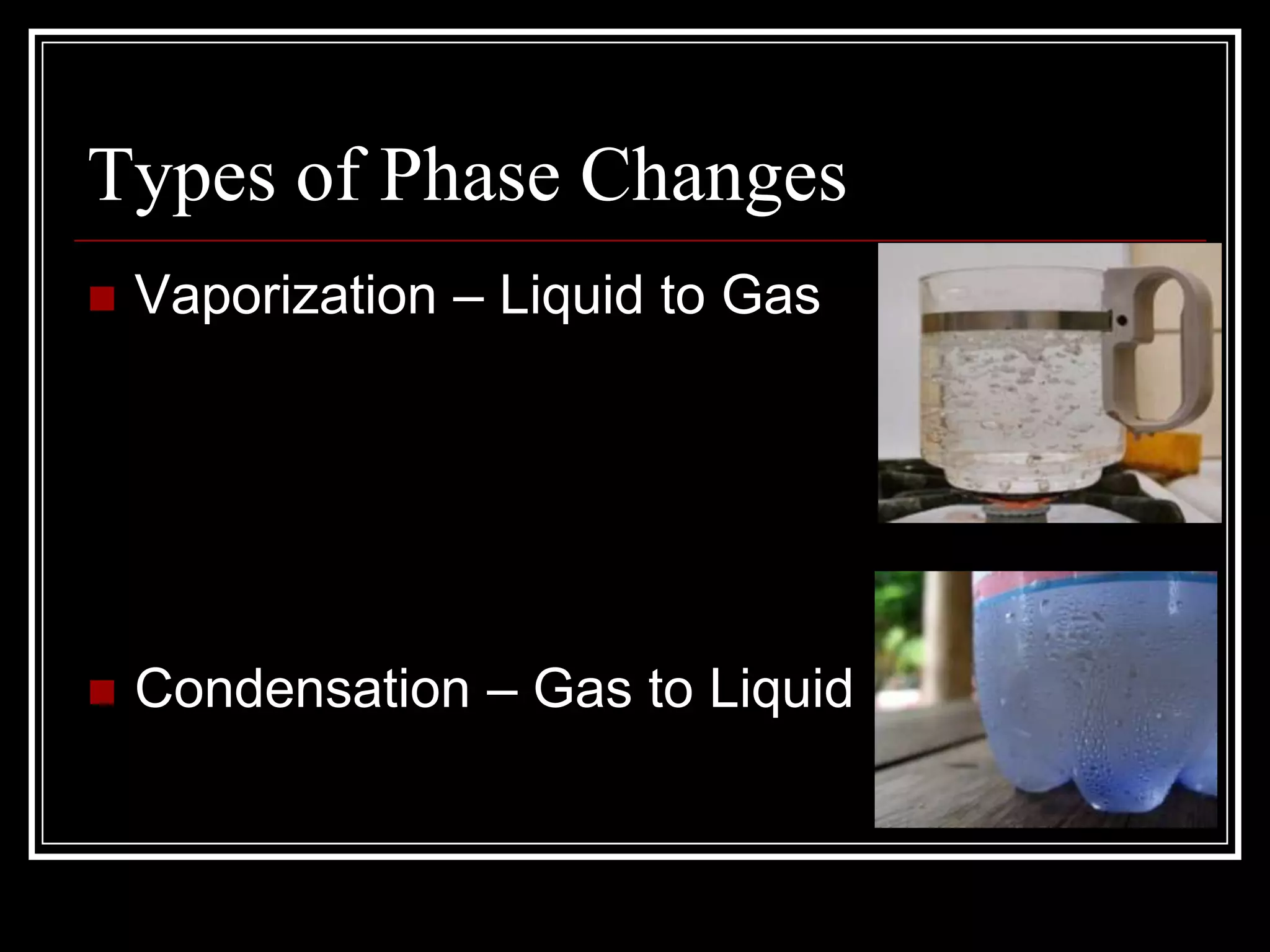
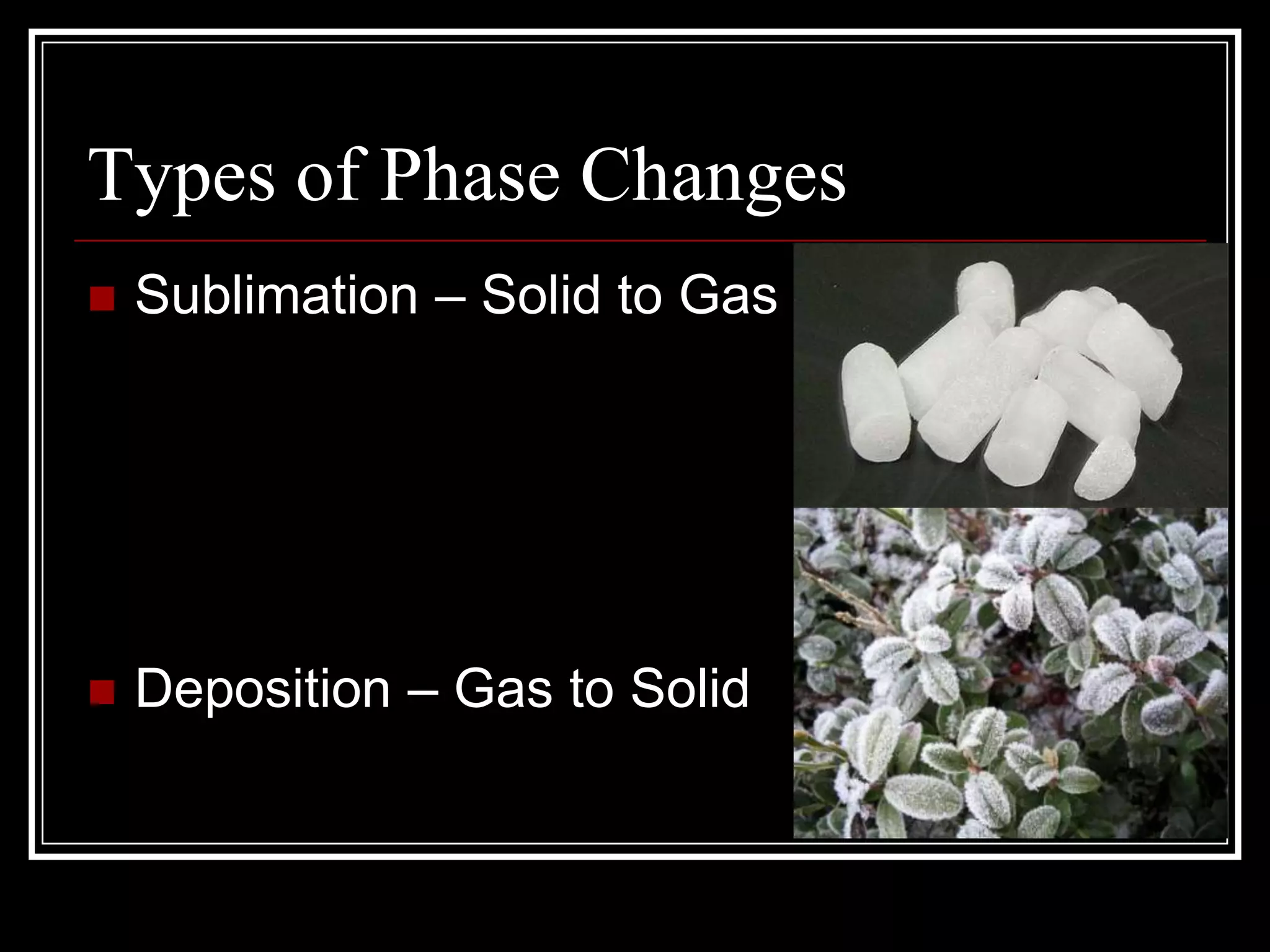


The document discusses the four phases of matter: solids, liquids, gases, and plasma. It describes the properties of each phase, including how tightly or loosely packed the particles are and whether they have a definite volume and shape. It also explains the different types of phase changes that can occur as matter transitions between phases, such as melting, freezing, boiling, condensing, sublimating, and depositing.










