Embed presentation
Download to read offline

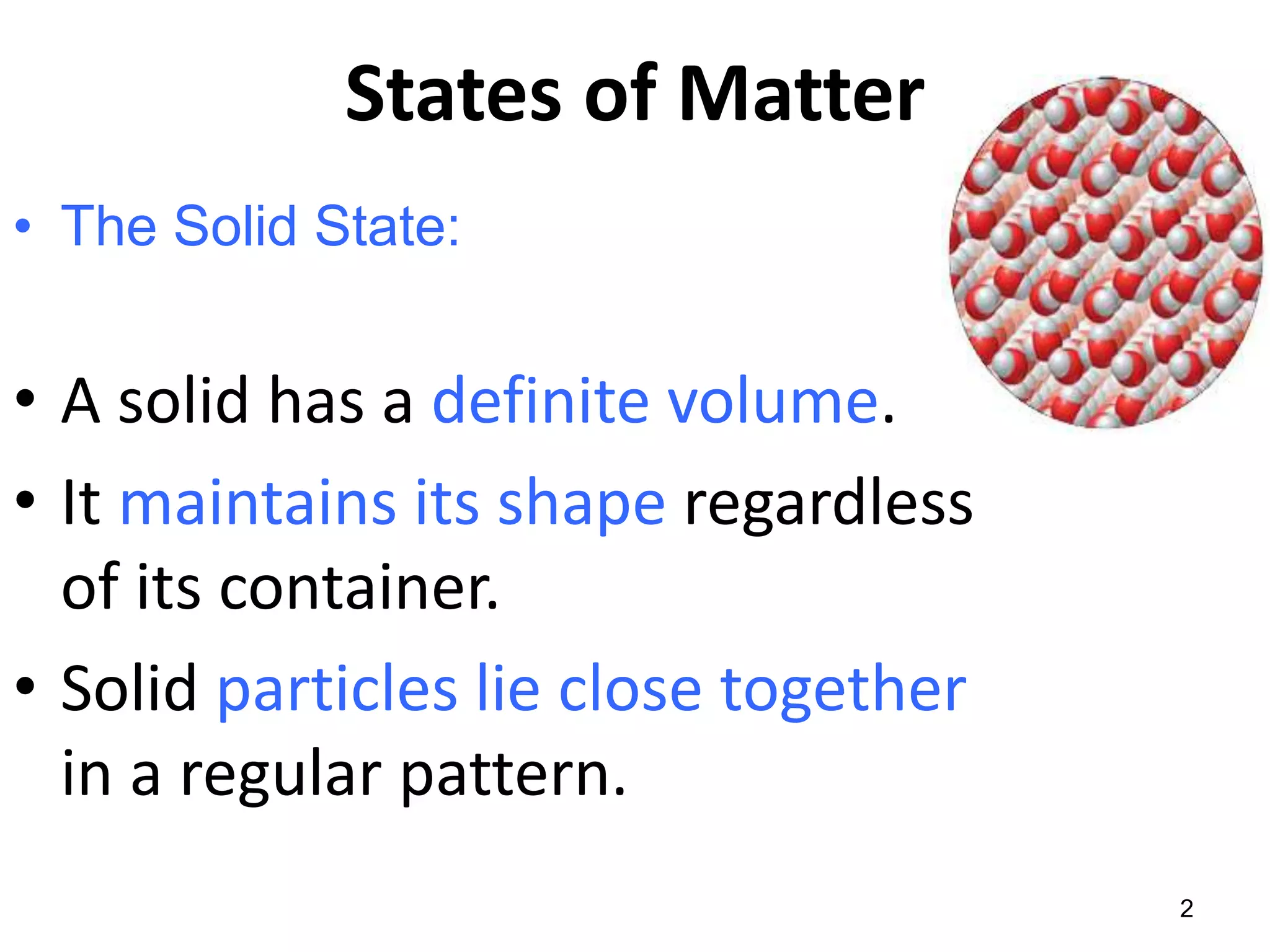
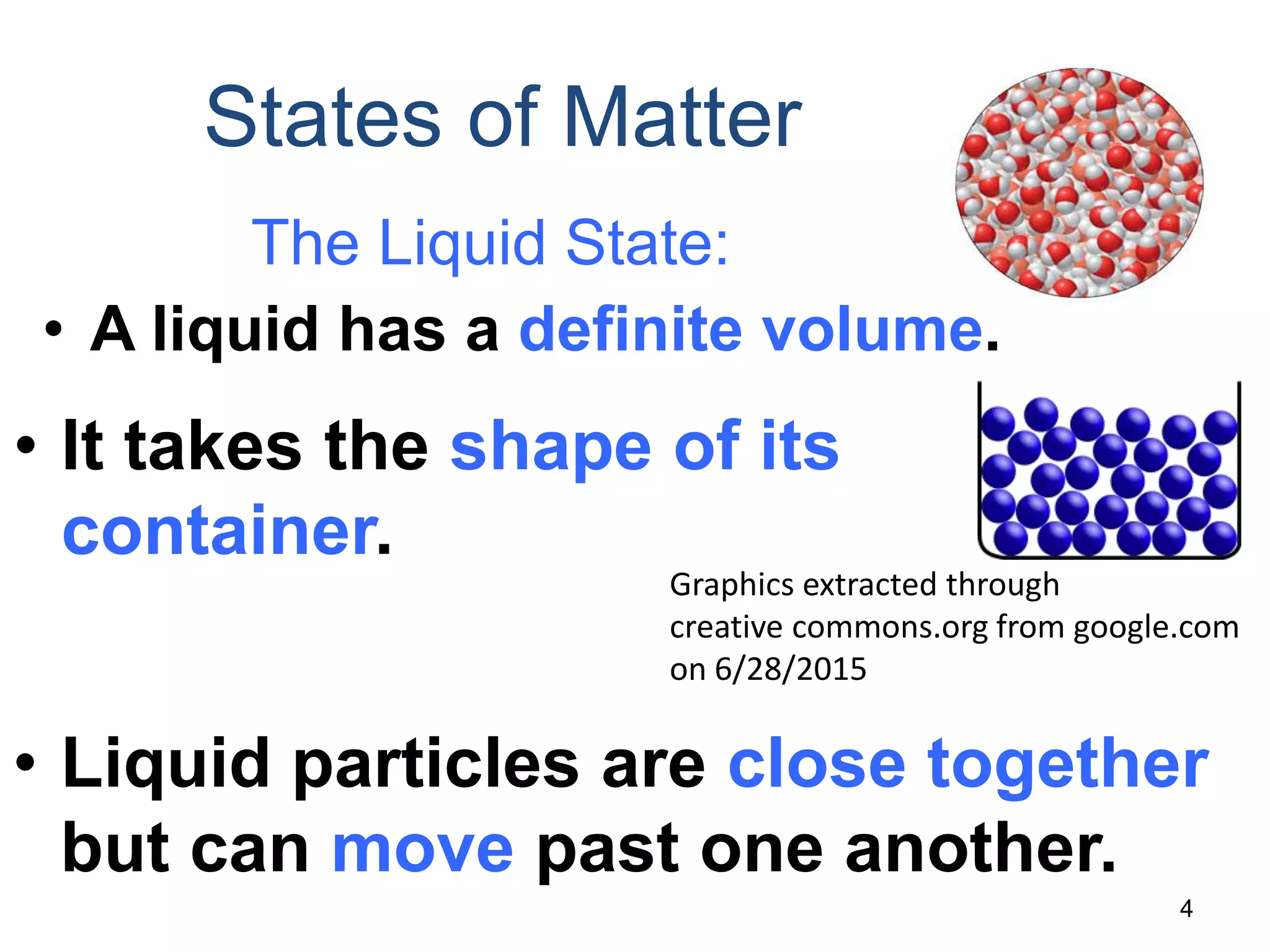
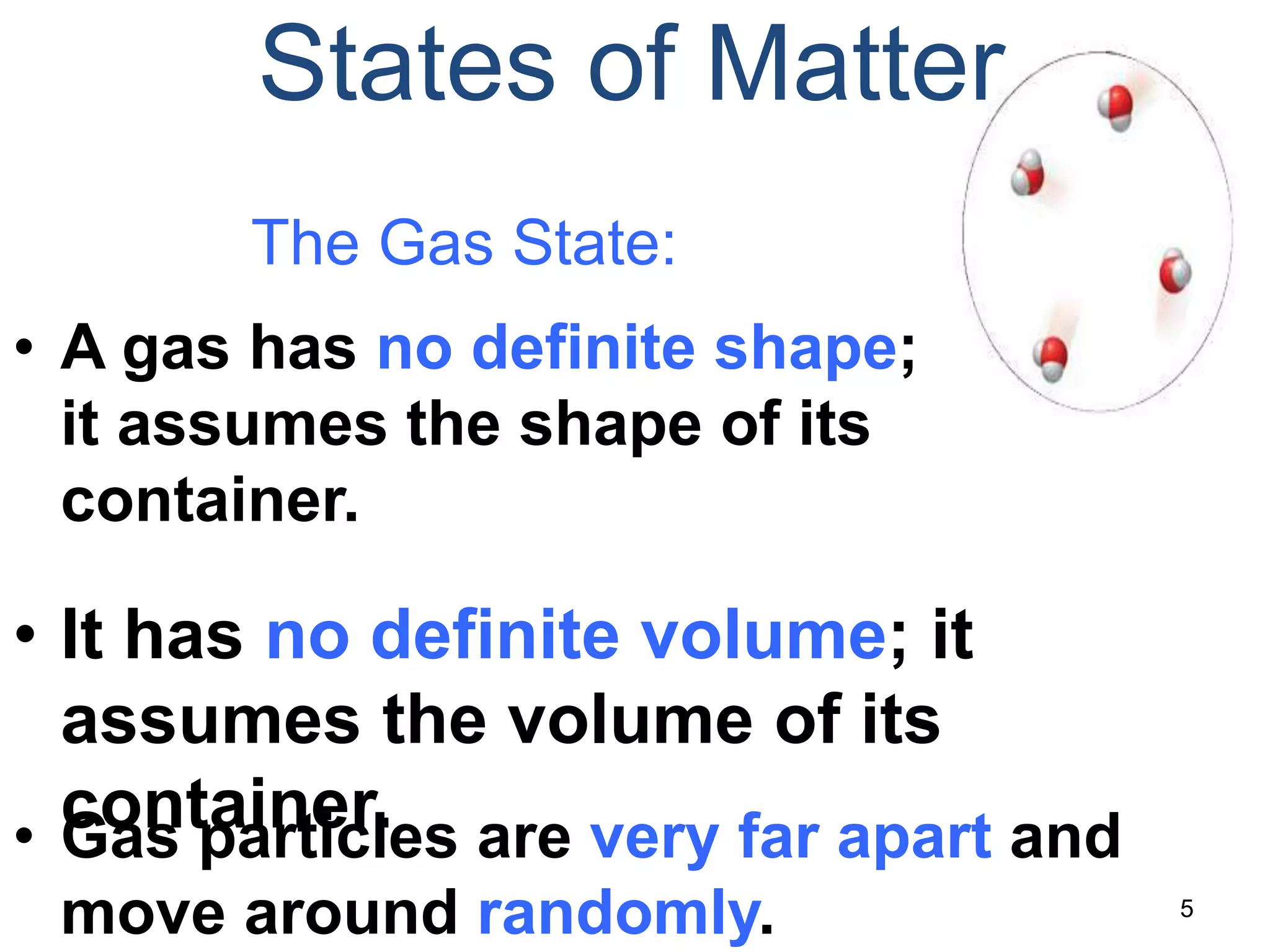
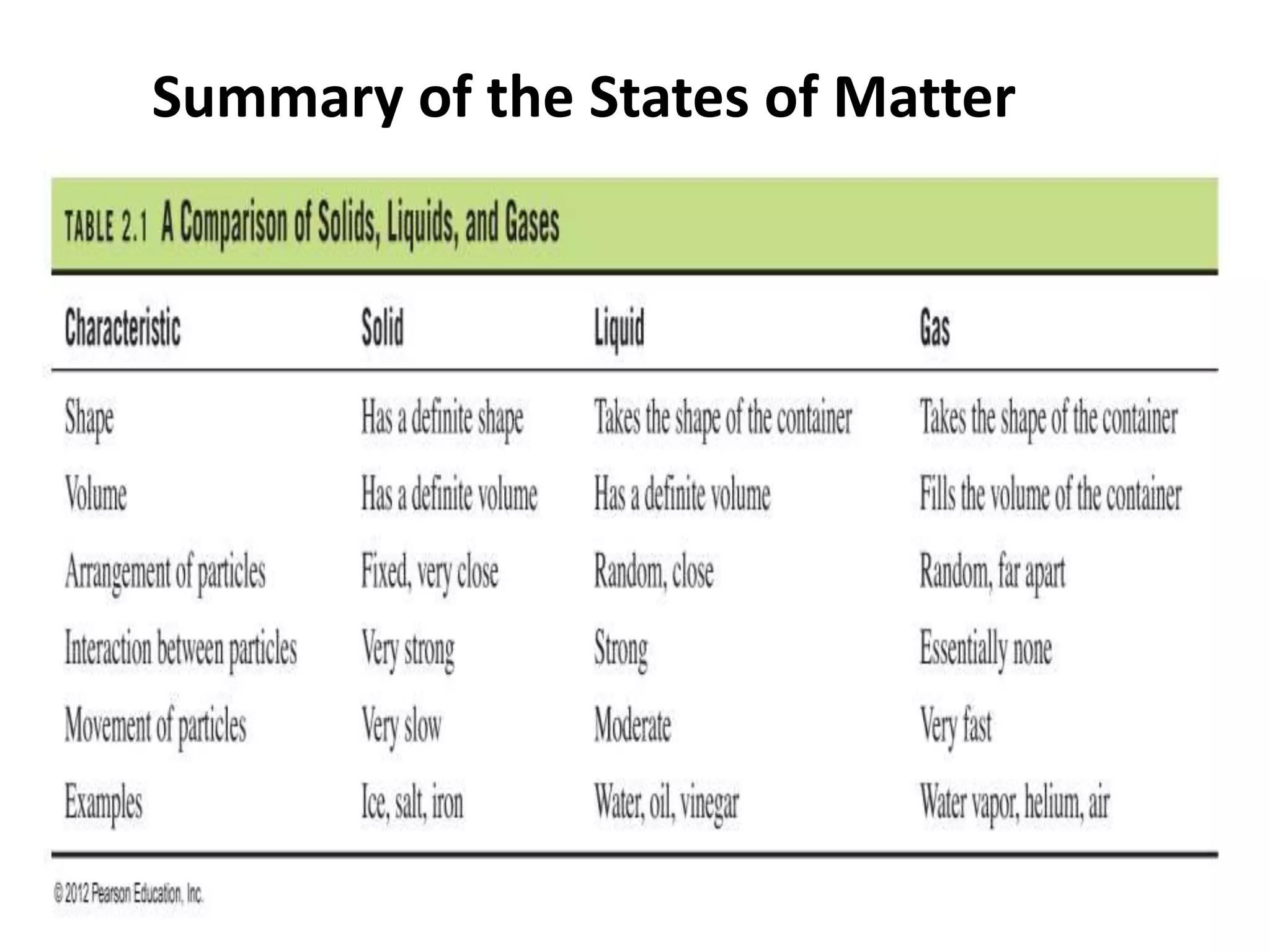


This document discusses the three states of matter - solid, liquid, and gas. It describes solids as having a definite volume and shape that does not change with the container. Solid particles are arranged in a regular pattern with little movement. Liquids also have a definite volume but take the shape of their container, and particles can move past one another. Gases have no definite volume or shape, filling their container completely, and particles are very far apart moving randomly.







