Embed presentation
Downloaded 21 times

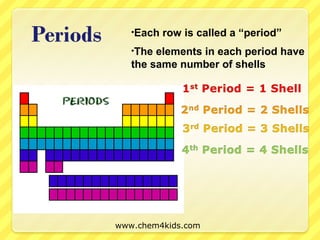
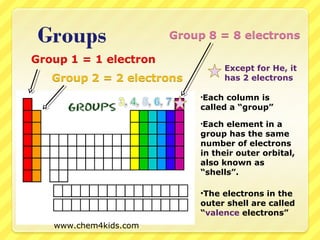
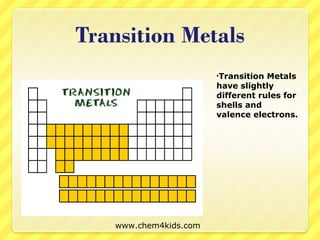
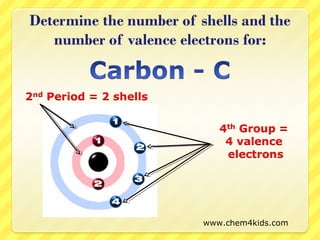
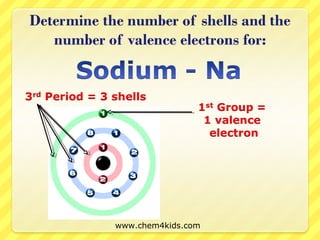







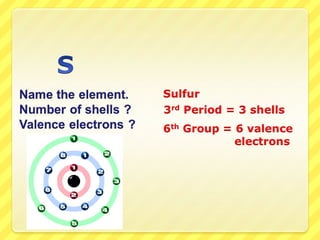

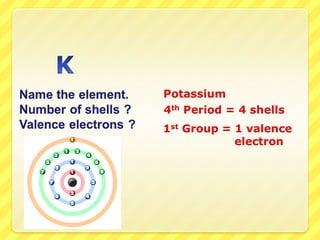


The document discusses the organization of the periodic table. It explains that elements are organized into periods based on the number of electron shells and into groups based on the number of electrons in the outer shell. Transition metals follow slightly different rules regarding shells and valence electrons. Helium, though in group 18, only has two electrons due to its single electron shell.

















