Embed presentation
Downloaded 44 times







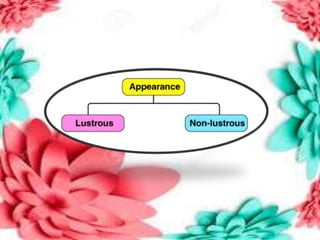




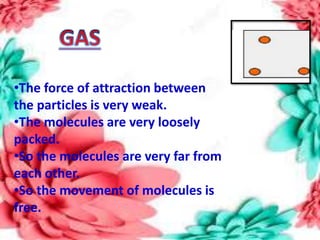








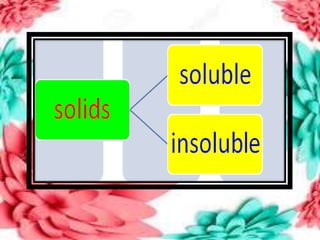


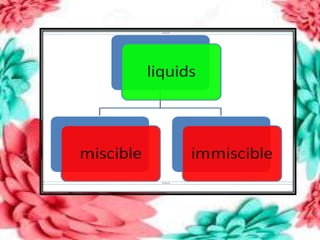



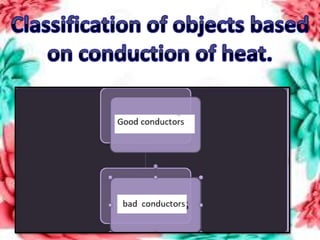












This document discusses the organization of objects and systematic study of them. It then describes three states of matter - solids, liquids, and gases - based on the strength of attraction between particles and how closely packed the molecules are. It also discusses miscibility of liquids and conductivity of materials based on their hardness.










































