1. The document discusses electrolysis and differentiates between conductors, electrolytes, and non-electrolytes based on their ability to conduct electricity and undergo chemical changes.
2. It describes the components of an electrolytic cell including the electrodes (anode and cathode) and explains that electrolysis is the process of decomposing an electrolyte into its constituent elements using an electric current.
3. The summary explains that during electrolysis, electric energy is converted to chemical energy as ions in the electrolyte migrate to the electrodes. At the anode, anions are discharged and at the cathode, cations are discharged.






![MODULE • Chemistry FORM 4
© Nilam Publication Sdn. Bhd. 108
5UNIT
Explanation / Penerangan:
– Potassium chloride solution consists of K+
, H+
, Cl–
and OH–
ions that move freely.
Larutan kalium klorida mengandungi ion K+
, H+
, Cl–
dan OH–
yang bergerak bebas.
– Cl–
ion and OH–
ion move to the anode.
Ion Cl–
dan ion OH–
bergerak ke anod.
– OH–
ion is lower than Cl–
ion in the Electrochemical Series.
Ion OH–
terletak di bawah ion Cl–
dalam Siri Elektrokimia.
– OH–
ion is selectively discharged by releasing electrons to form oxygen and water molecules.
Ion OH–
dipilih untuk dinyahcaskan dengan melepaskan elektron membentuk molekul oksigen dan air .
– Half equation / Persamaan setengah: 4OH–
2H2O + O2 + 4e .
– H+
ion is lower than K+
ion in the Electrochemical Series.
Ion H+
terletak di bawah ion K+
dalam Siri Elektrokimia.
– H+
ion is selectively discharged by receiving electrons to form hydrogen molecules.
Ion H+
dipilih untuk dinyahcaskan dengan menerima elektron membentuk molekul hidrogen .
– Half equation / Persamaan setengah: 2H+
+ 2e H2
.
3 Describe an experiment to determine the product of electrolysis copper(II) sulphate solution with carbon electrode. Your answer
should include the observation, confirmatory test for the product at the anode and half equation at the electrode.
Huraikan satu eksperimen untuk menentukan hasil elektrolisis larutan kuprum(II) sulfat menggunakan elektrod karbon. Dalam
jawapan anda perlu disertakan pemerhatian, ujian pengesahan untuk hasil yang terbentuk di anod dan persamaan setengah
bagi tindak balas yang berlaku di elektrod.
Answer / Jawapan:
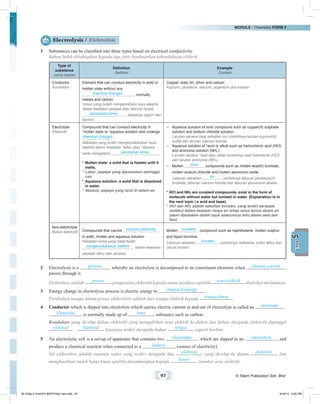
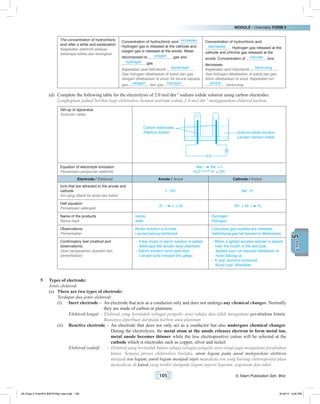
Apparatus / Radas : Battery/powersupply,carbonelectrodes,wire,electrolyticcell,testtube,Ammeter[fromalabelleddiagram]
Bateri/bekalan kuasa, elektrod karbon, wayar, sel elektrolisis, tabung uji, ammeter (dari rajah berlabel)
Material / Bahan : 1 mol dm–3
copper(II) sulphate solution / Larutan kuprum(II) sulfat 1 mol dm–3
Carbon electrodes
Elektrod karbon
Copper(II) sulphate solution
Larutan kuprum(II) sulfat
Procedure / Langkah:
(a) Pour 1 mol dm–3
copper(II) sulphate solution in the electrolytic cell until it is half full .
Masukkan larutan kuprum(II) sulfat 1 mol dm–3
ke dalam sel elektrolisis sehingga separuh penuh .
(b) The apparatus is set up as shown in the diagram. Fill the test tube with copper(II) sulphate solution and invert
the test tube on the anode .
Radas disusunkan seperti dalam gambar rajah. Isi tabung uji dengan larutan kuprum(II) sulfat dan terbalikkan
tabung uji itu pada anod .
(c) Carbon electrodes are connected to batteries using connecting wire.
Elektrod karbon disambung kepada bateri menggunakan wayar penyambung.
(d) Collect the gas produced at the anode .
Kumpulkan gas yang terhasil di anod .
(e) Gas produced at the anode is tested with a glowing wooden splinter .
Gas yang terhasil di anod diuji dengan kayu uji berbara .
05 Chap 5 ChemF4 Bil(FSY5p) new.indd 108 9/18/15 5:05 PM](https://image.slidesharecdn.com/unit5elektrokimia-160807005935/85/Unit-5-elektrokimia-13-320.jpg)