The document discusses several models of atomic structure:
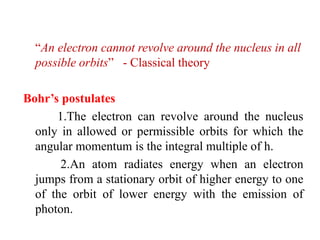
1. Bohr's model, which postulated that electrons orbit the nucleus in allowed orbits with angular momentum that is an integer multiple of Planck's constant. This model could calculate orbital radii and energies.
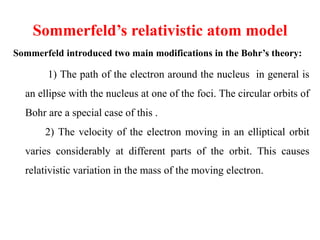
2. Sommerfeld's relativistic model improved on Bohr by allowing elliptical orbits and accounting for relativistic effects on electron mass.
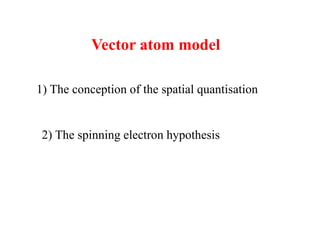
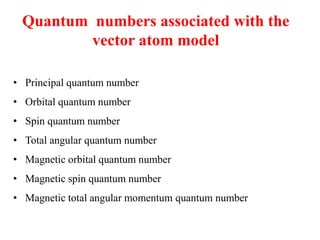
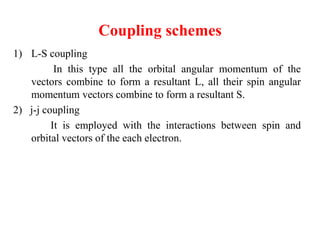
3. The vector model introduced spatial quantization and the spinning electron hypothesis to explain phenomena like the Zeeman and Stark effects. It associated quantum numbers with angular momentum.
4. Pauli's exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers.