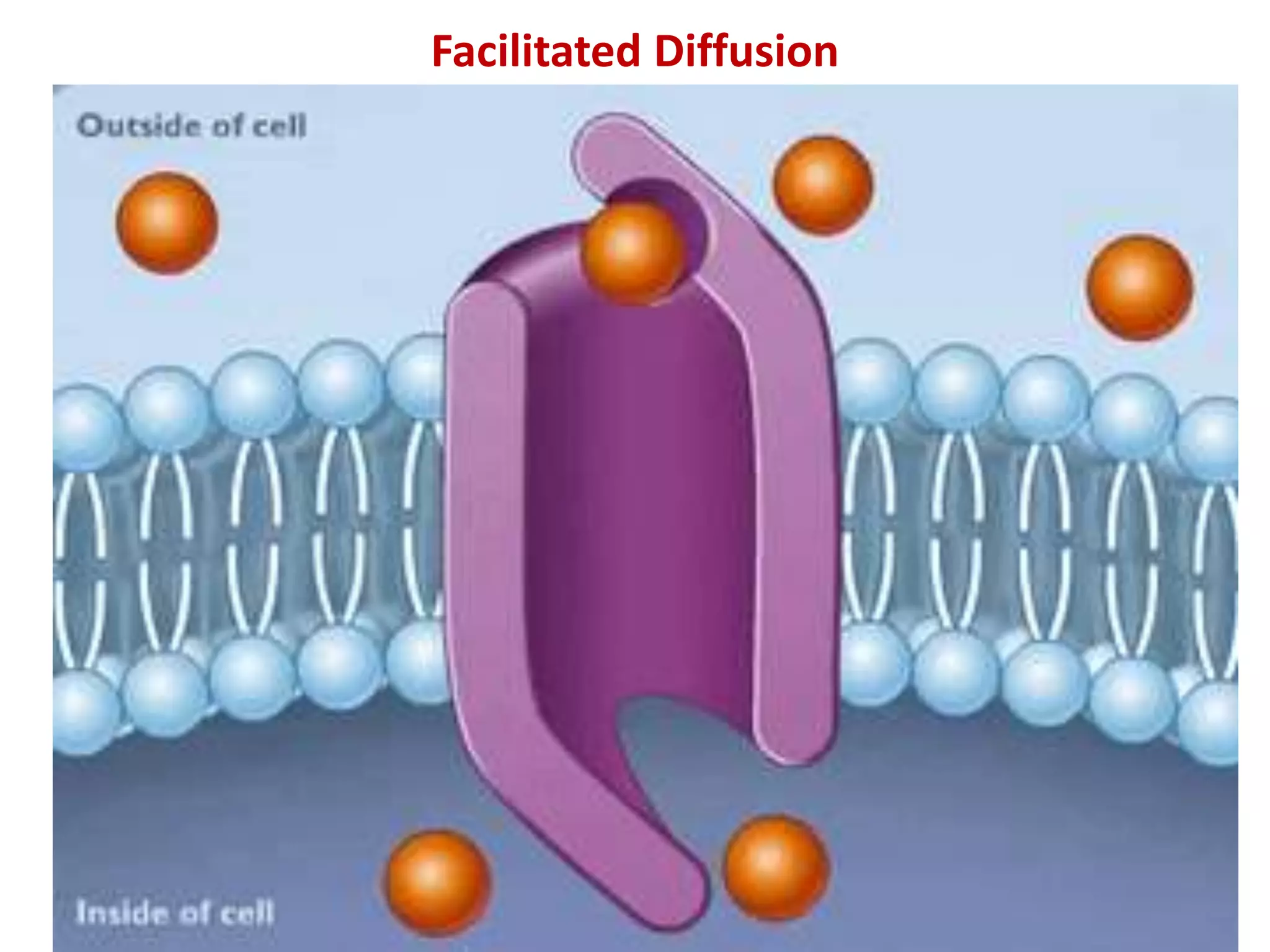
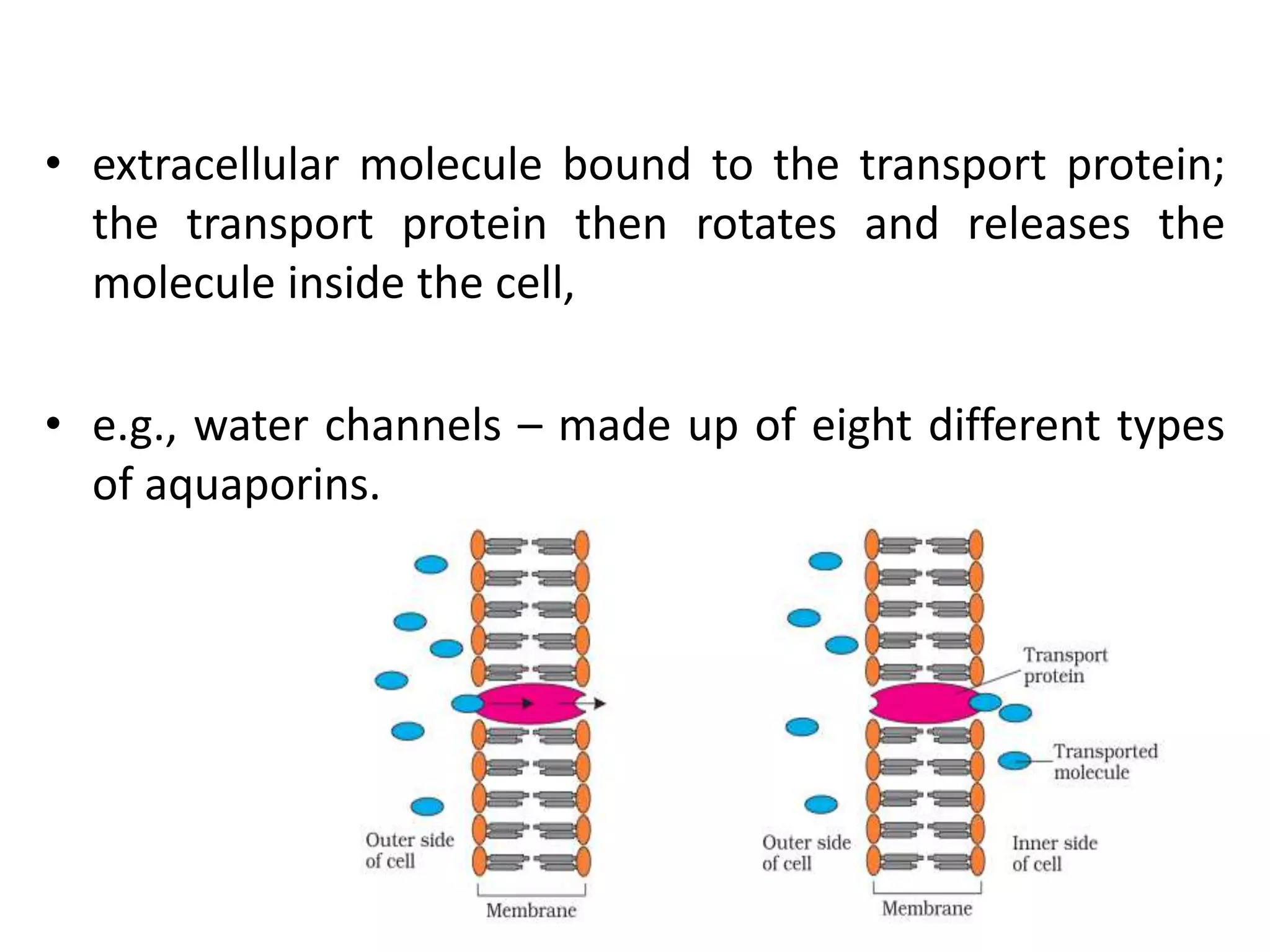
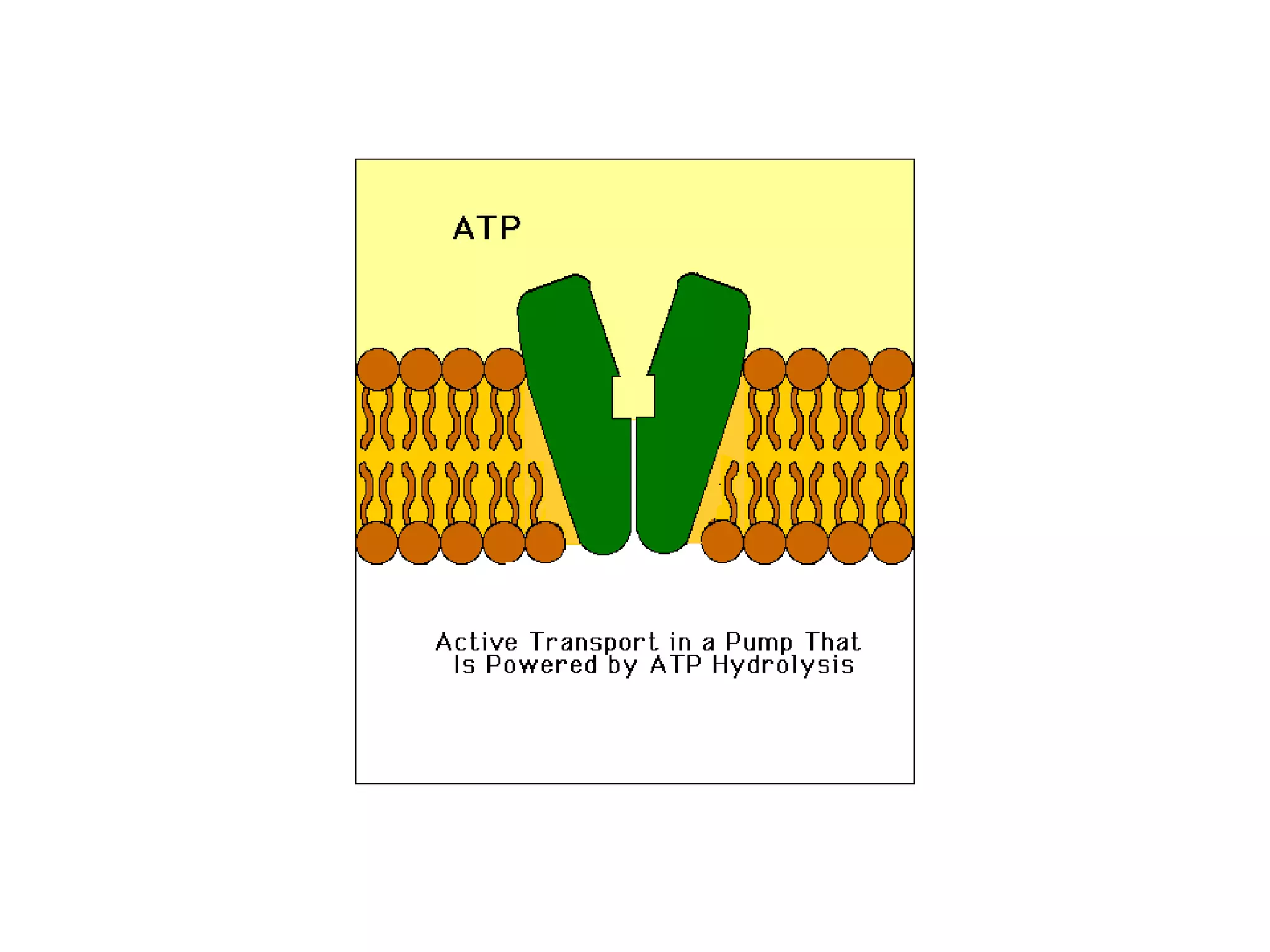
This document discusses various modes of transport in plants including diffusion, facilitated diffusion, active transport, and transport in xylem and phloem. Diffusion is the passive movement of substances from areas of higher concentration to lower concentration without expending energy. Facilitated diffusion uses membrane proteins to transport substances across membranes. Active transport uses energy to pump molecules against a concentration gradient using carrier proteins like ion pumps. Xylem transports water and minerals unidirectionally from roots to stems while phloem transports nutrients bidirectionally.