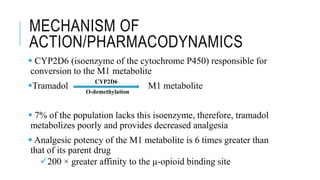
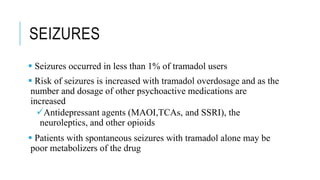
Tramadol is a centrally-acting analgesic with a dual mechanism of action, binding weakly to μ-opioid receptors and inhibiting the reuptake of norepinephrine and serotonin. It has fewer side effects than other opioids like morphine and a lower risk of abuse and dependence. Tramadol is effective for moderate to moderately severe pain and does not affect the prostaglandin cycle like NSAIDs. While less potent than morphine, tramadol has a better side effect profile and safety margin at therapeutic doses.

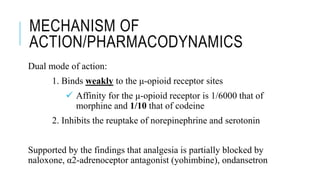
![MECHANISM OF
ACTION/PHARMACODYNAMICS
Tramadol is a synthetic 4-phenyl-piperidine analogue of
codeine
Chemical name is cis-2-[(dimethylamino)methyl]-1-(3-
methoxyphenyl) cyclohexanol hydrochloride
Parent compound is a racemic drug, and both its (+) and
(−) forms play an important role in its mechanism
The (+) enantiomer has a higher affinity for the μ-receptor
and increases serotonin levels
The (−) enantiomer increases norepinephrine levels
(1R,2R)- & (1S,2S)-Tramadol
Enantiomers](https://image.slidesharecdn.com/tramadol-200612093426/85/Tramadol-8-320.jpg)