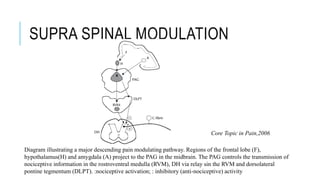
This document discusses neuropathic pain, its definition, symptoms, pathophysiology, assessment, and management. Some key points:
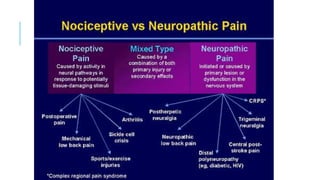
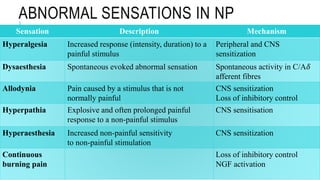
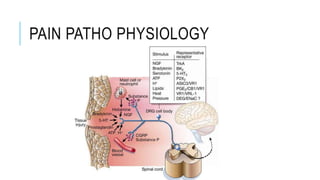
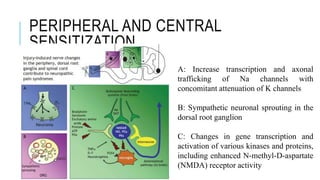
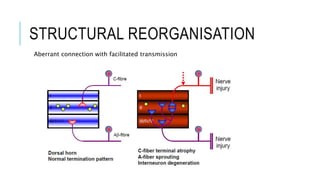
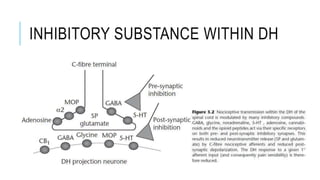
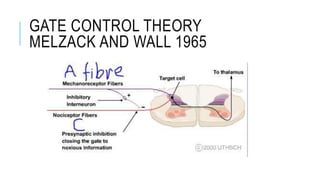
- Neuropathic pain is caused by damage or disease affecting the somatosensory nervous system. It is characterized by spontaneous ongoing pain, abnormal sensations, and hypersensitivity.
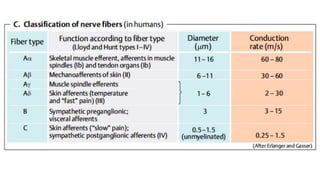
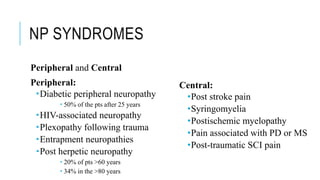
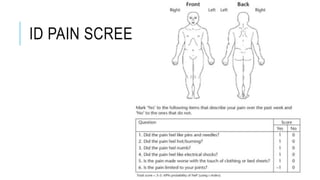
- Common causes include diabetic neuropathy, postherpetic neuralgia, spinal cord injury. Assessment involves history, exam, and tools like LANSS and DN4.
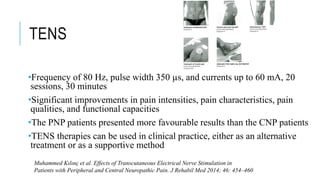
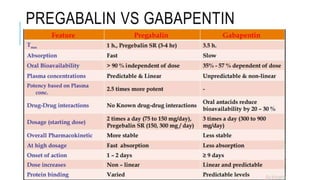
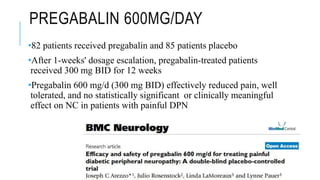
- Management includes non-pharmacological options like TENS, physical therapy, as well as drugs like gabapentin, pregabalin, tricyclic antidepressants.
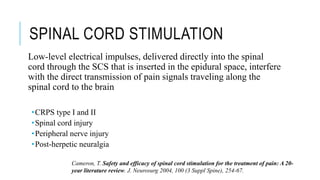
- For severe cases, neurosurgical options like cord