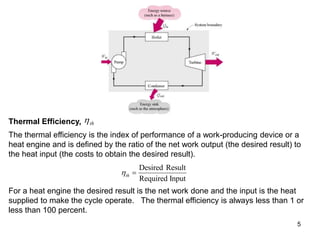
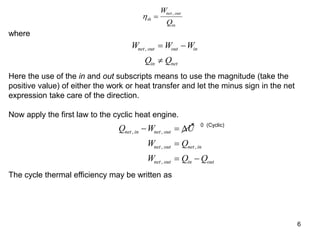
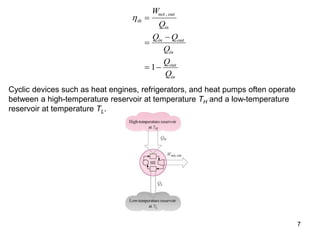
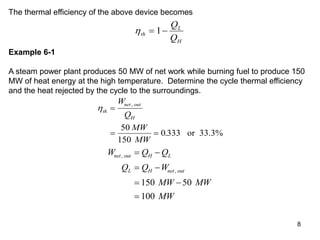
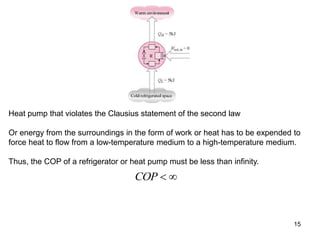
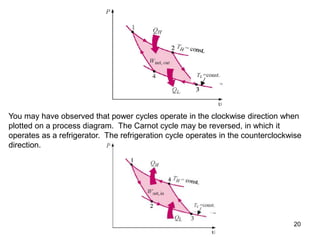
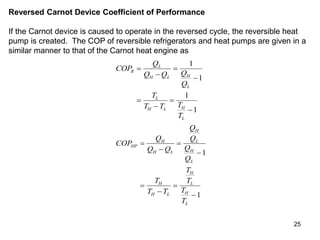
1) The Carnot cycle consists of two isothermal and two adiabatic processes between a high-temperature and low-temperature heat reservoir. It represents the most efficient reversible heat engine.
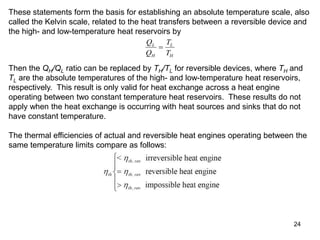
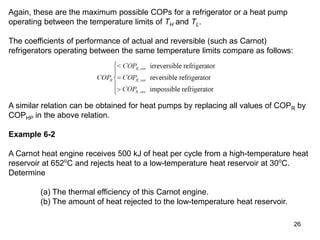
2) The Carnot principles state that no heat engine can be more efficient than a reversible Carnot engine operating between the same temperatures, and all reversible engines between the same temperatures have the same efficiency.
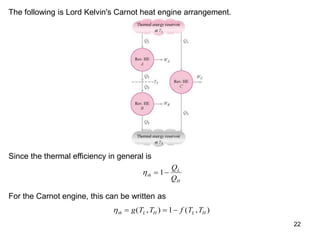
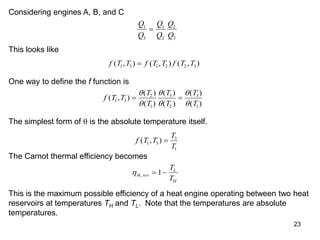
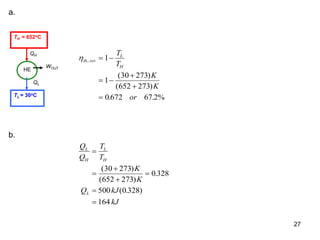
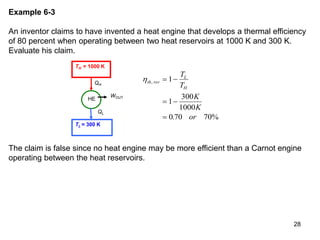
3) Lord Kelvin used the Carnot cycle to define an absolute temperature scale where the temperature ratio between reservoirs equals the efficiency of a Carnot engine between those reservoirs.