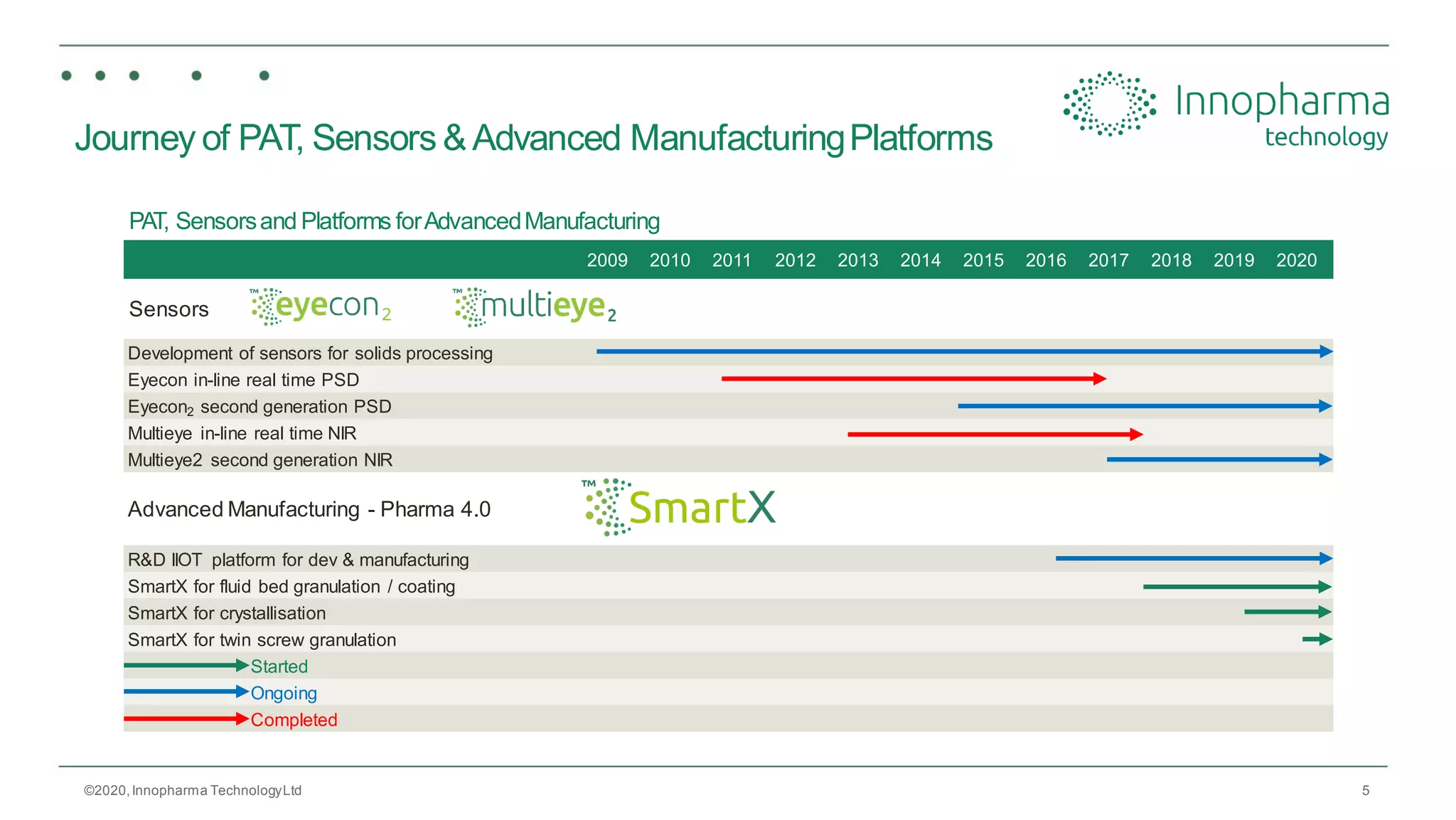
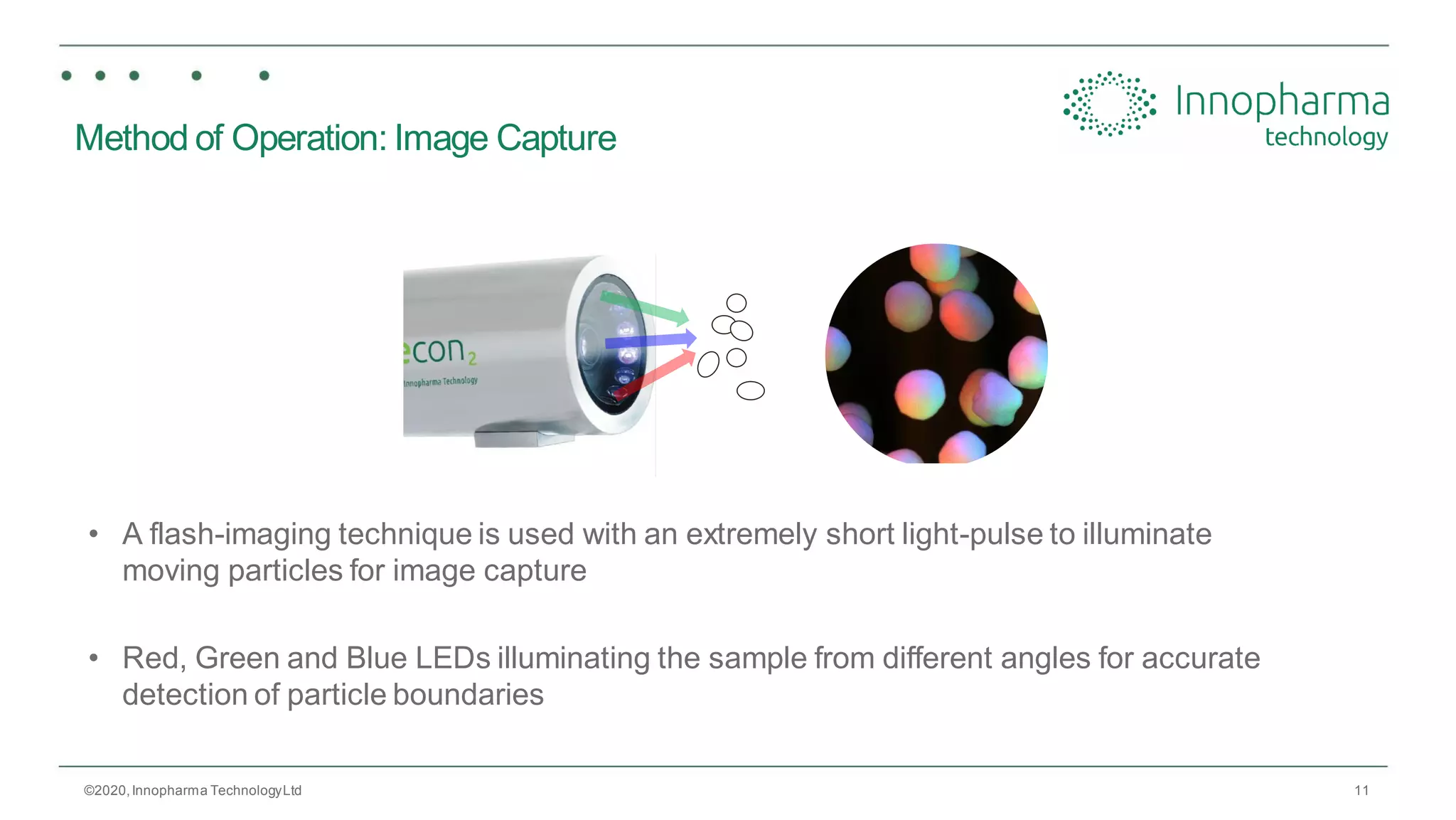
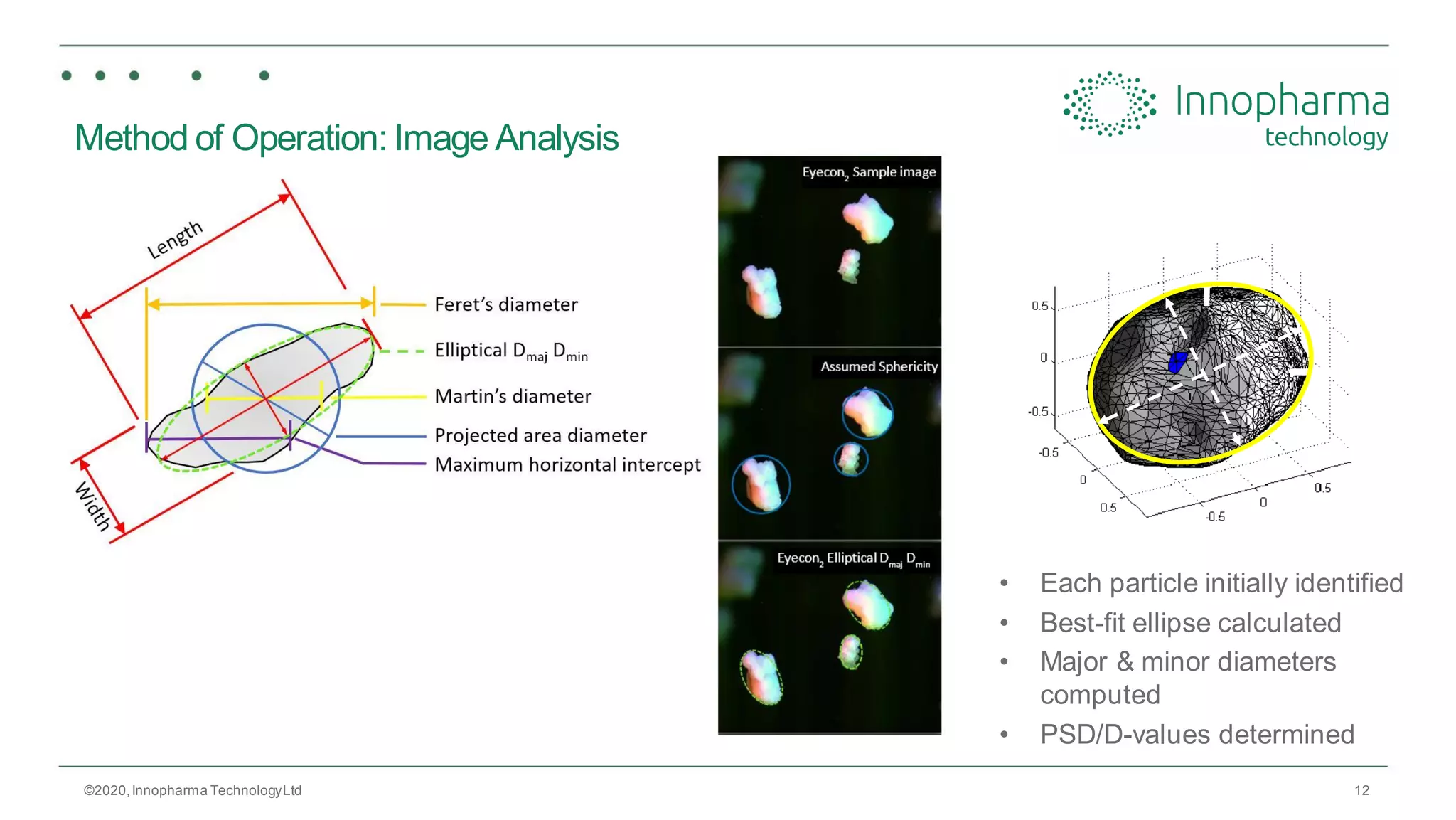
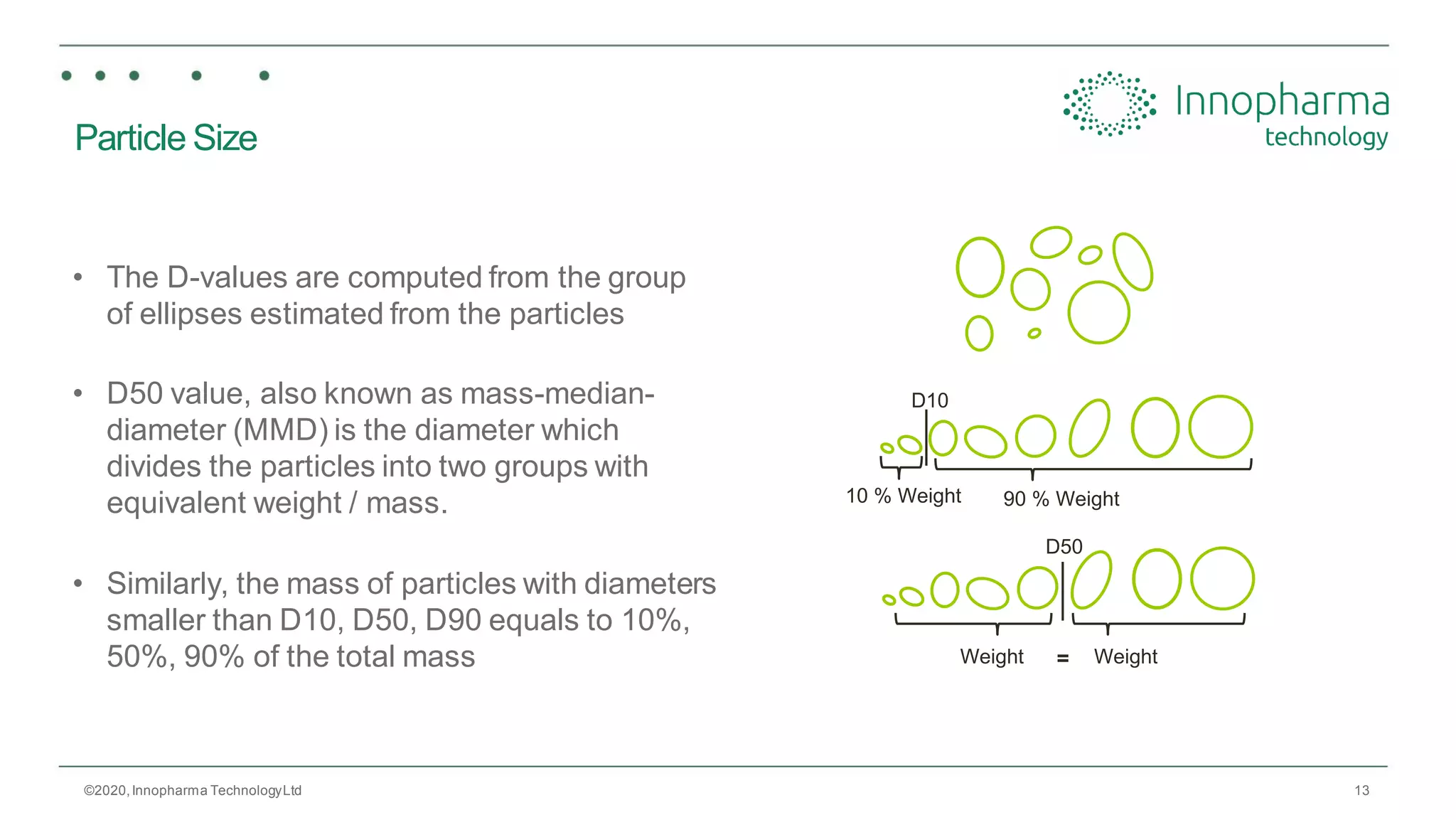
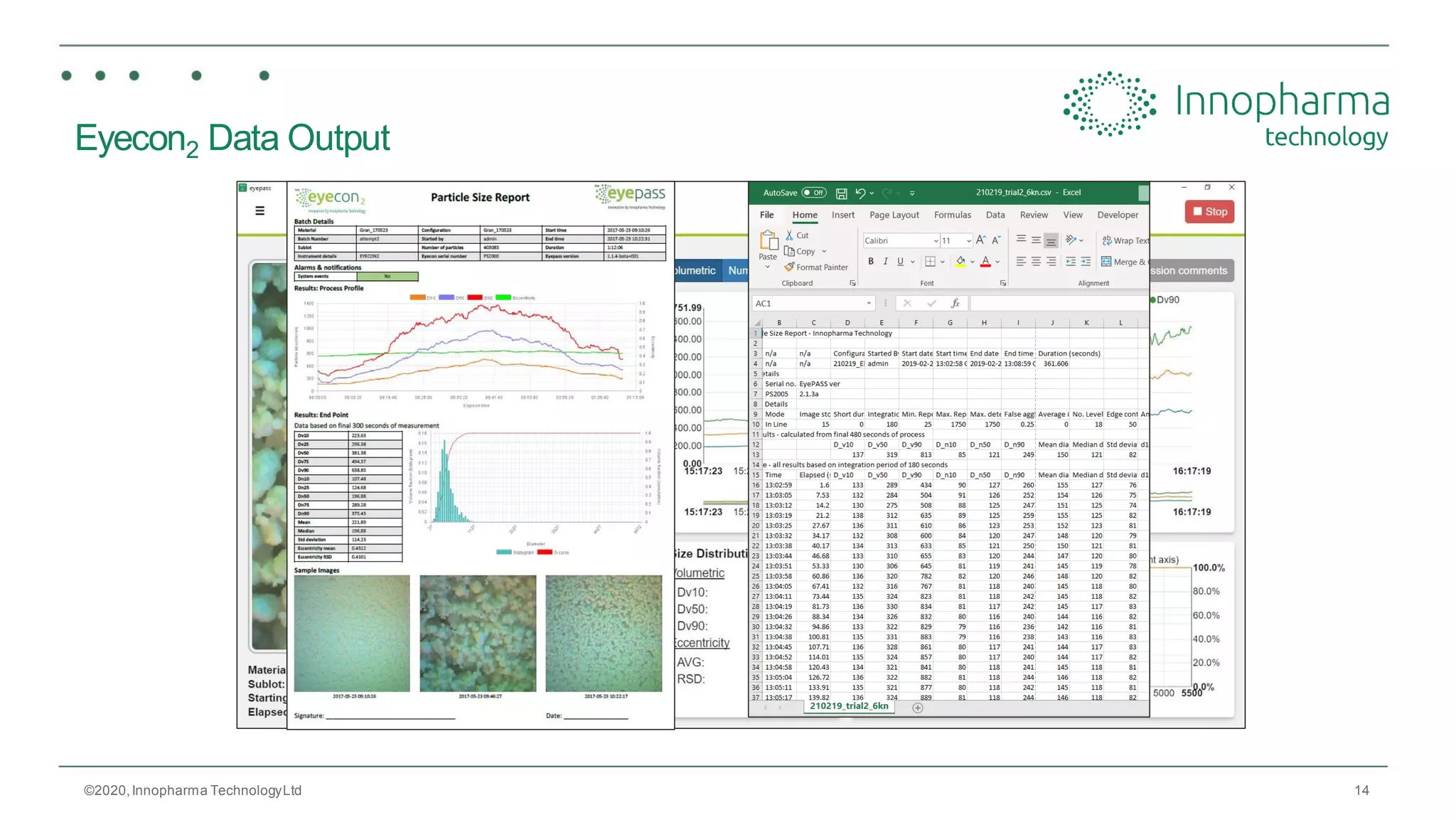
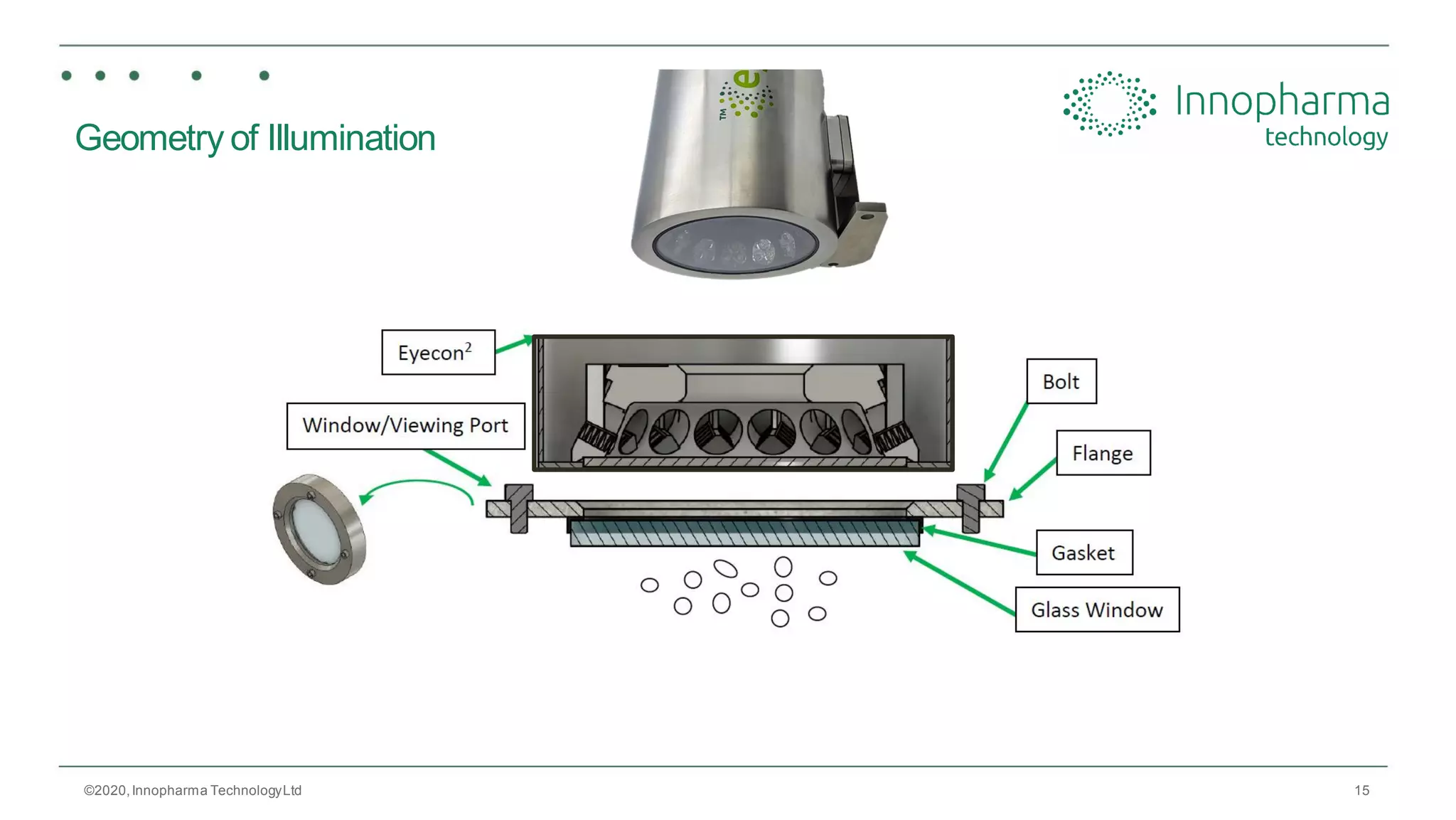
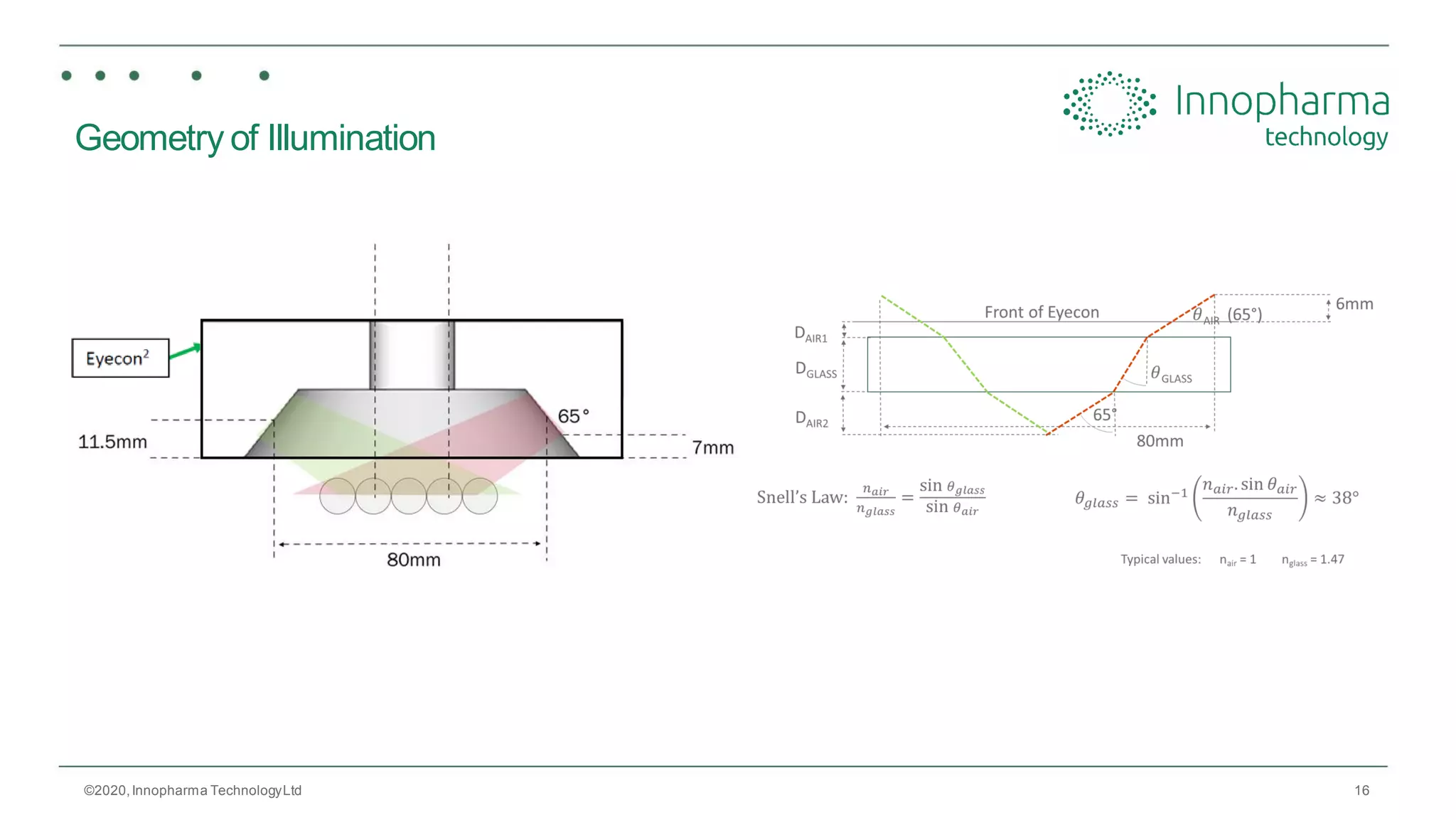
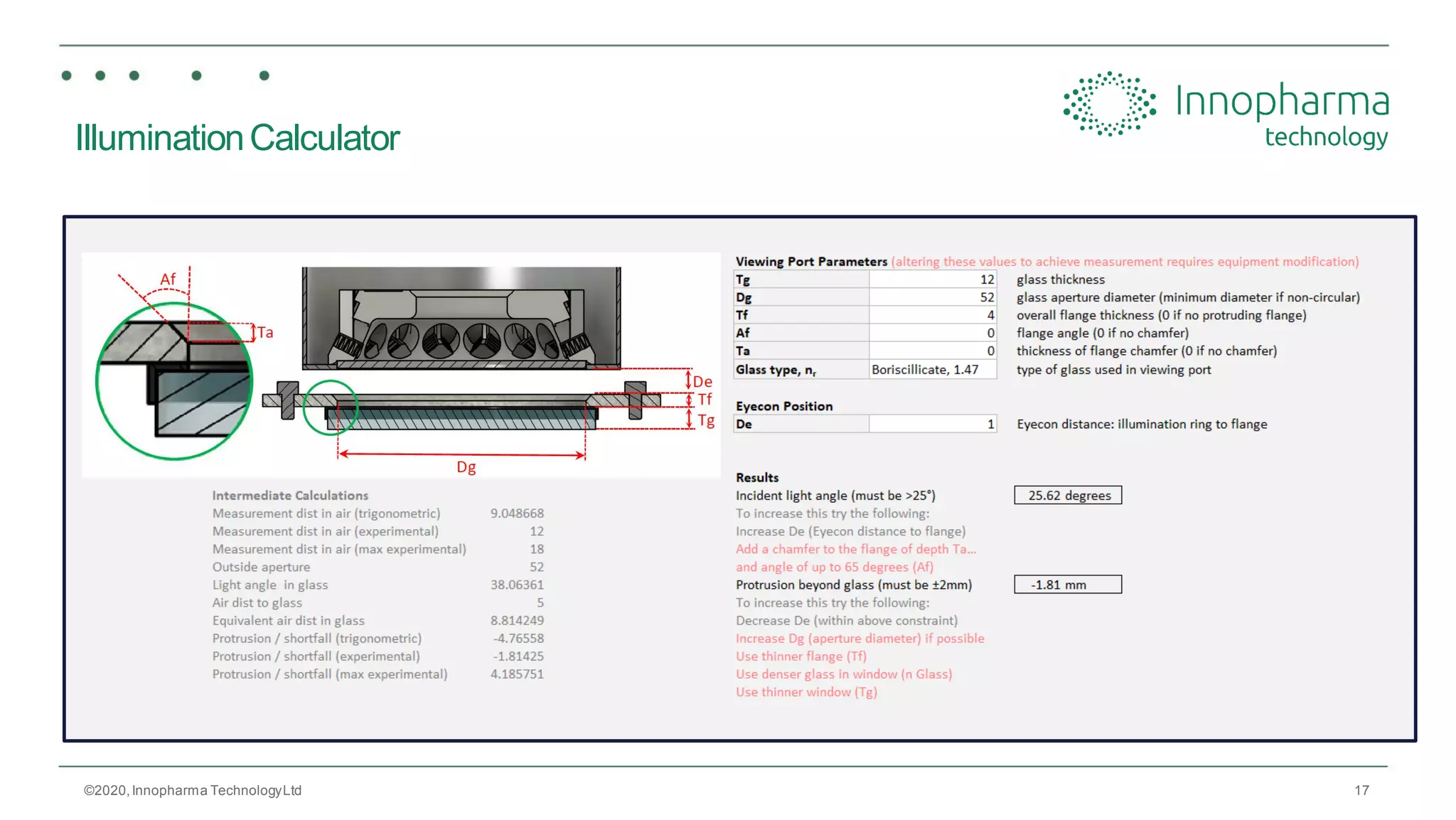
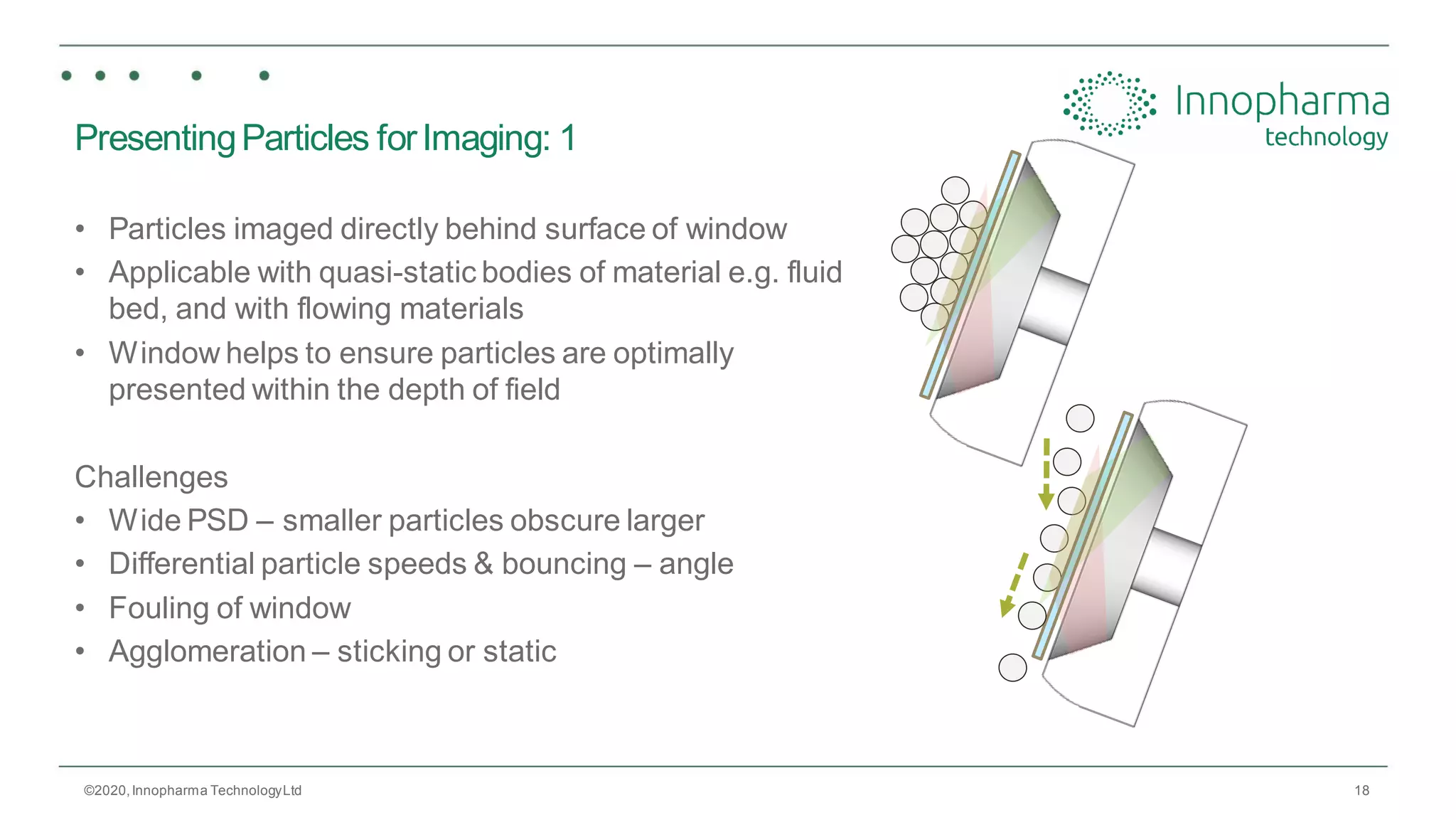
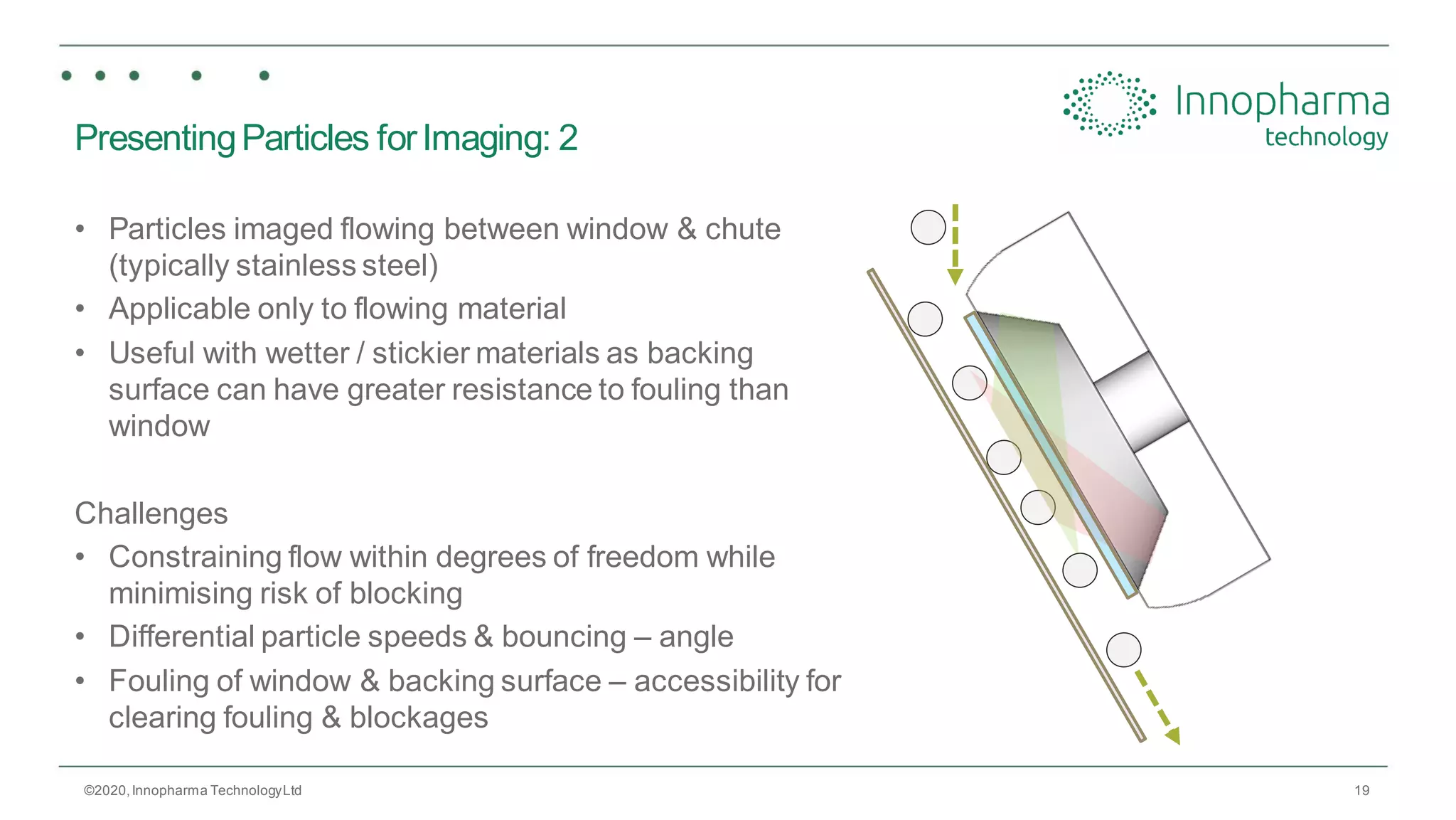
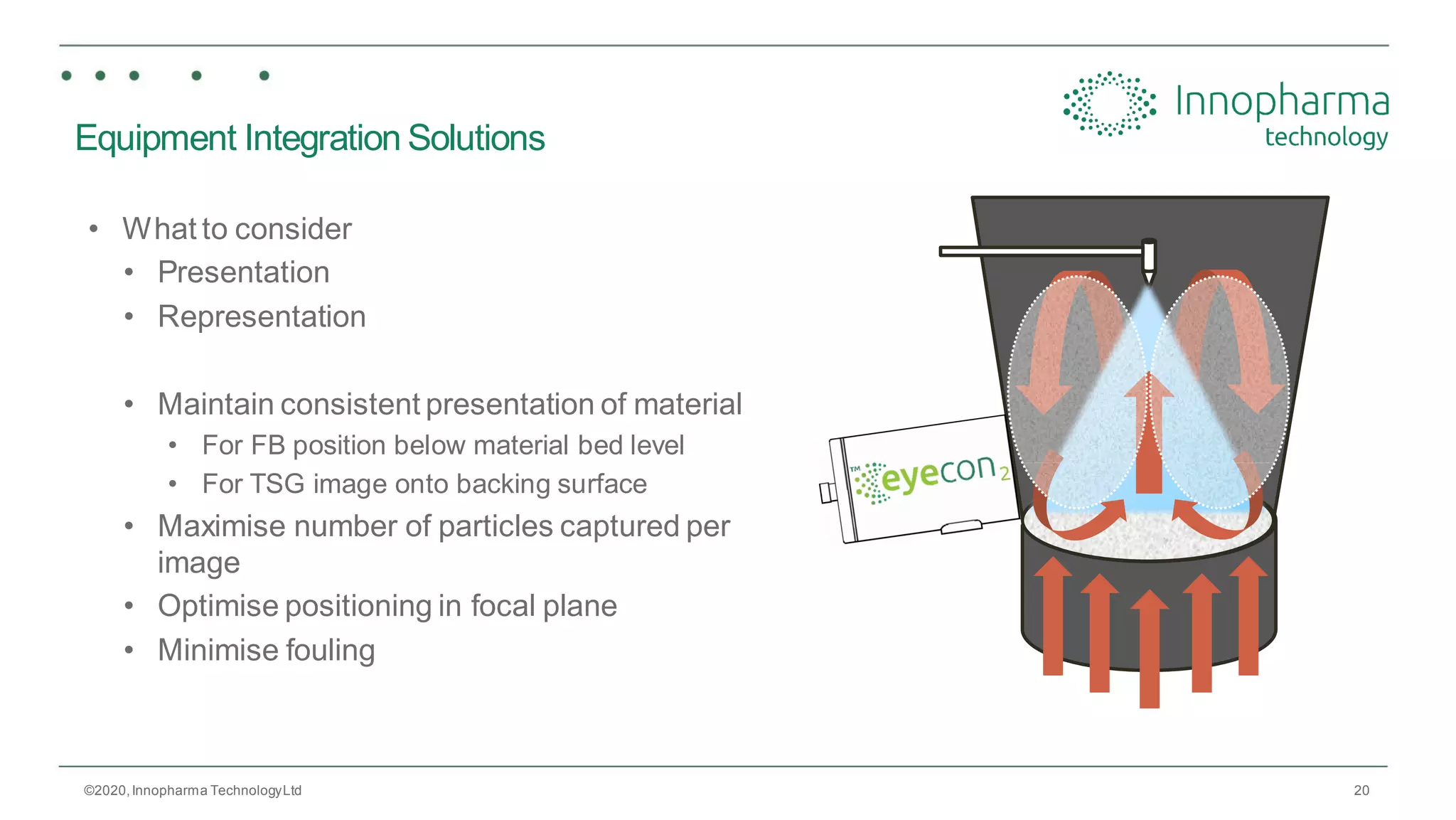
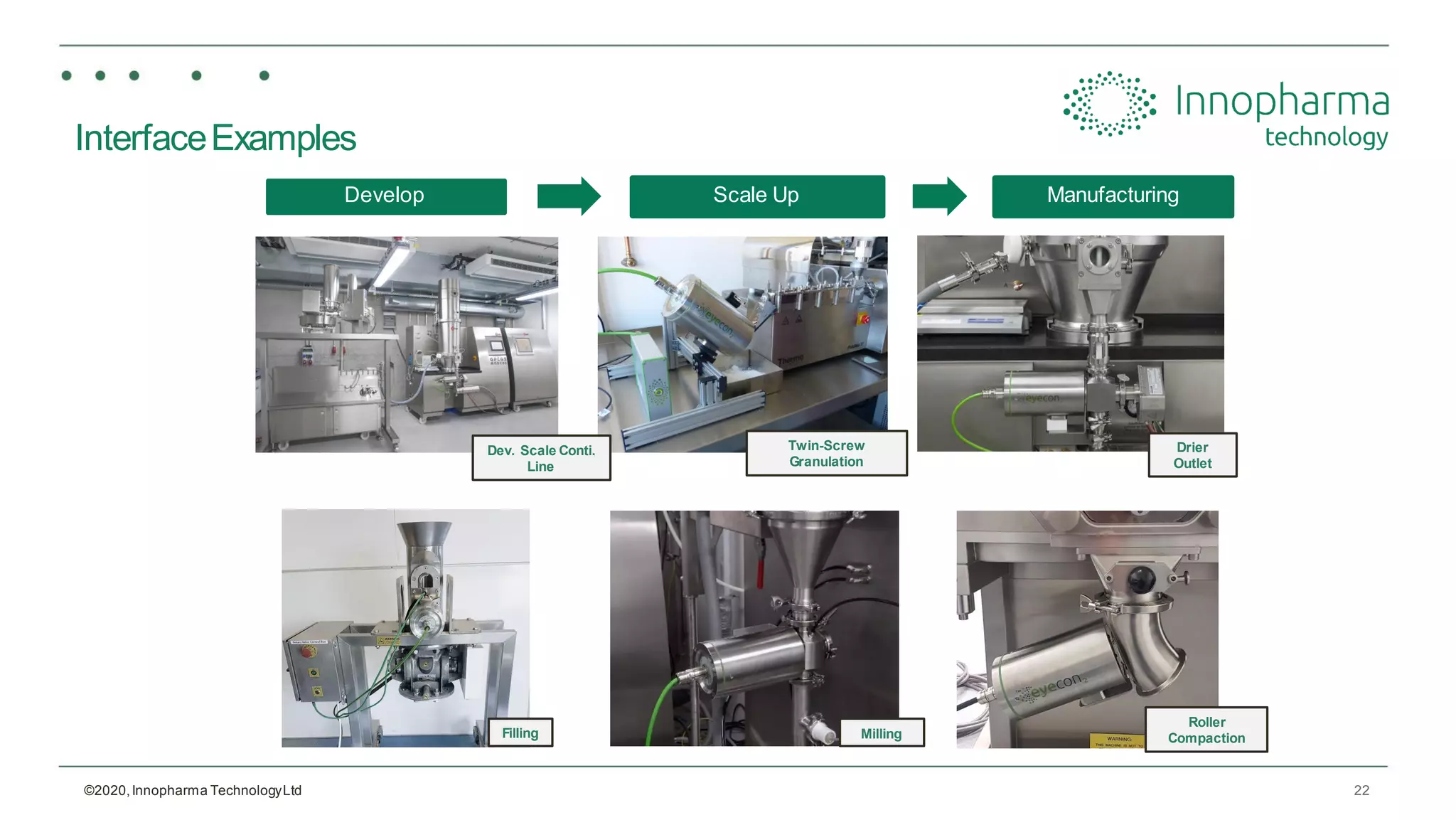
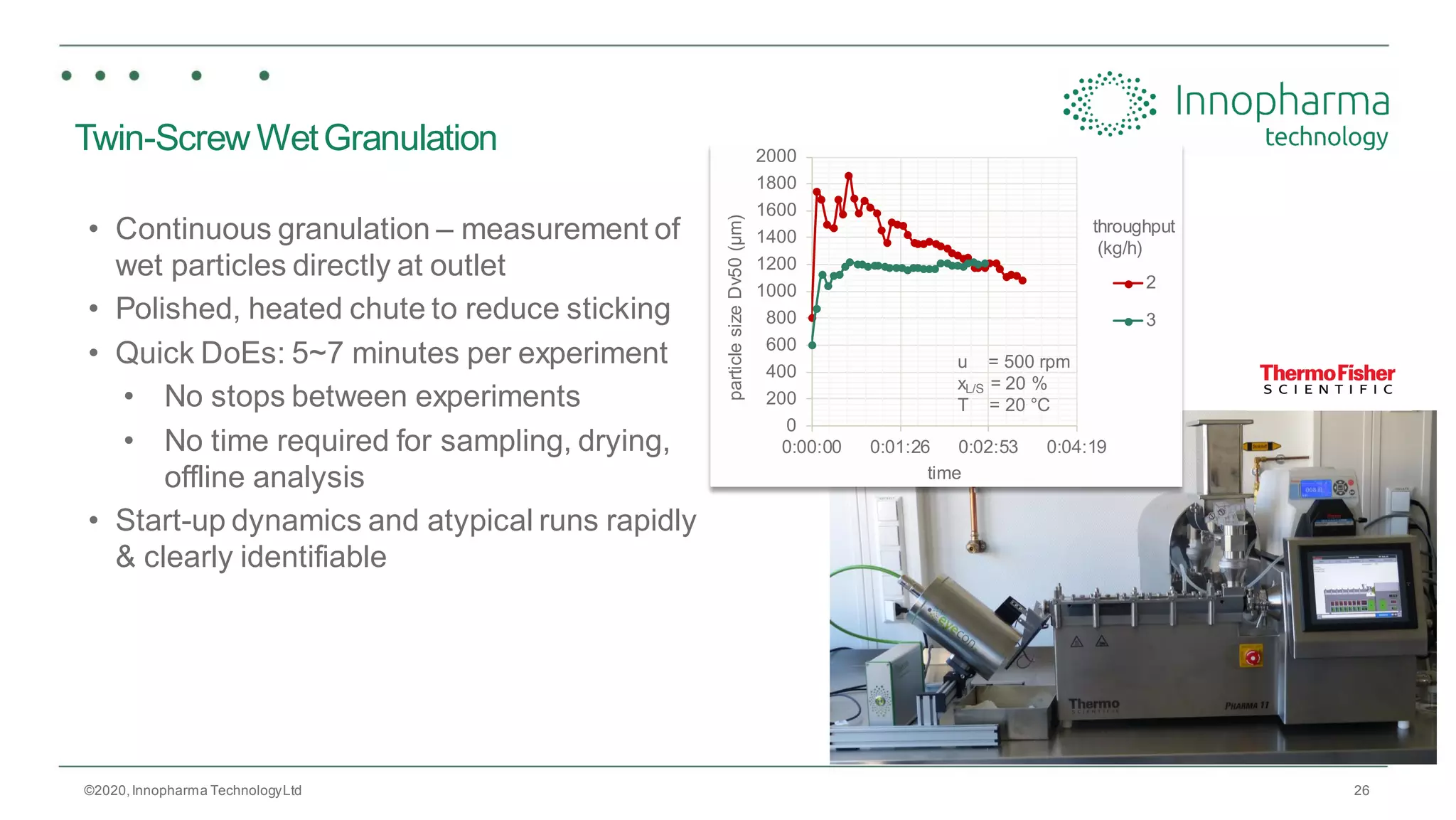
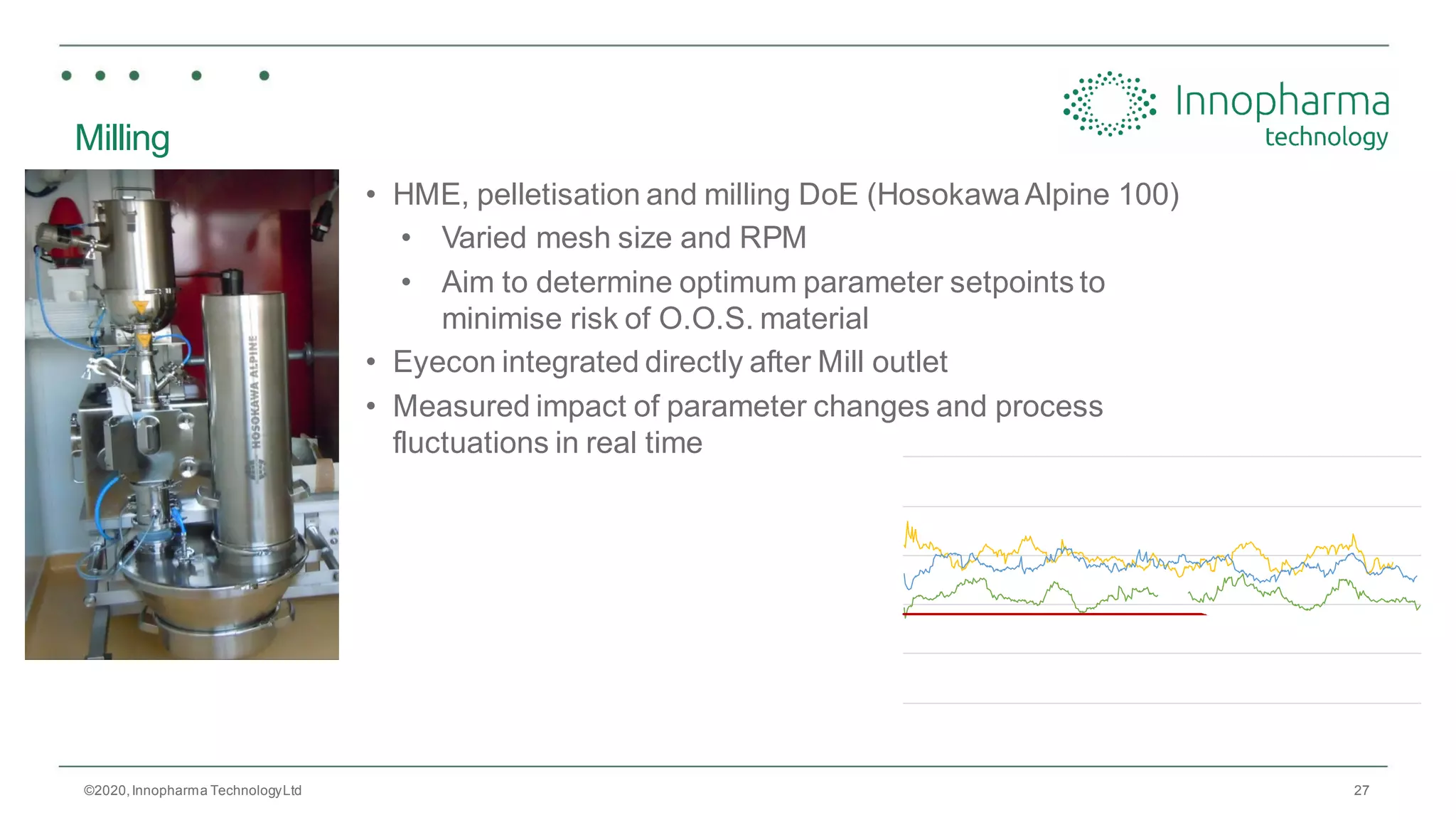
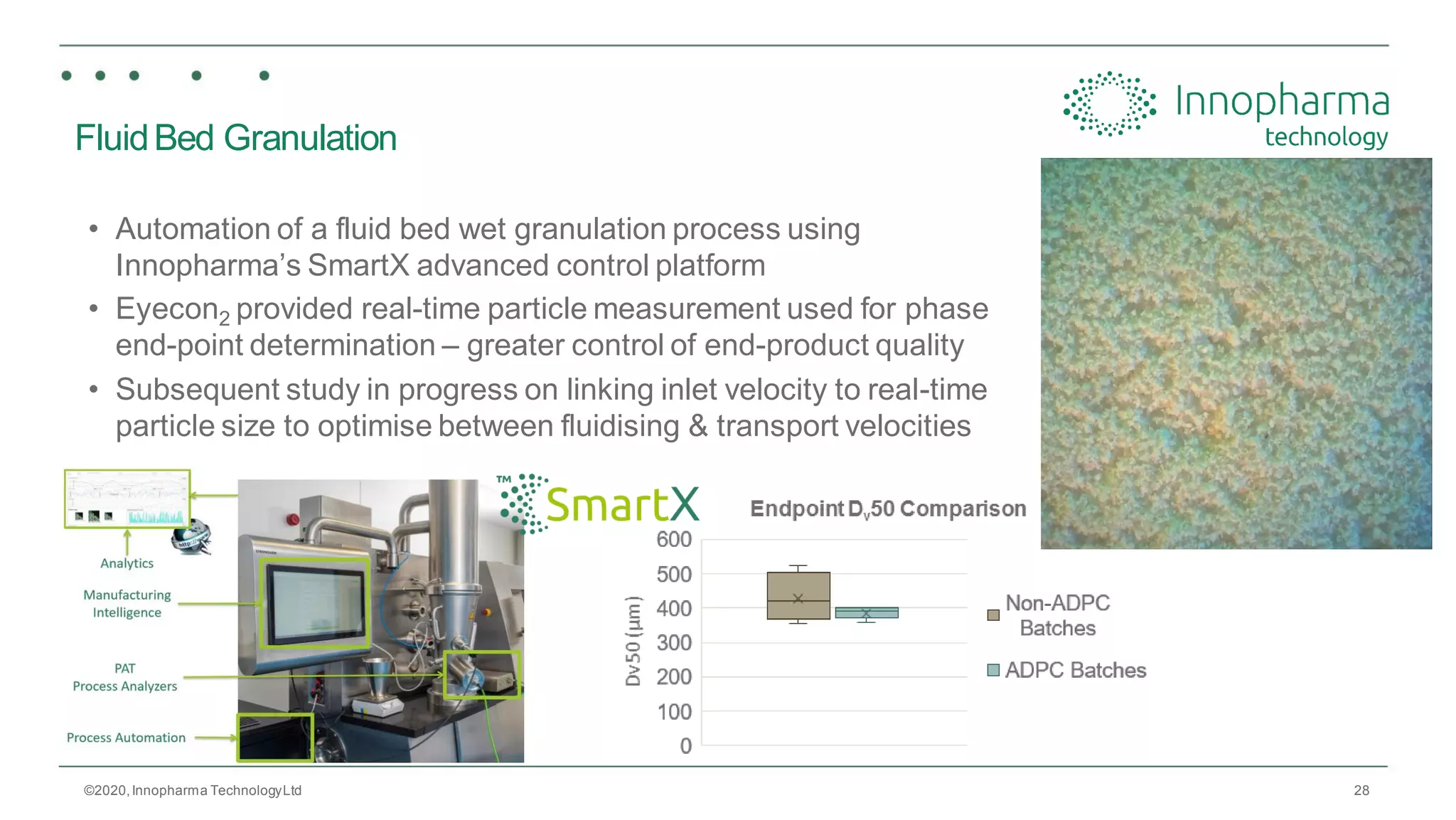
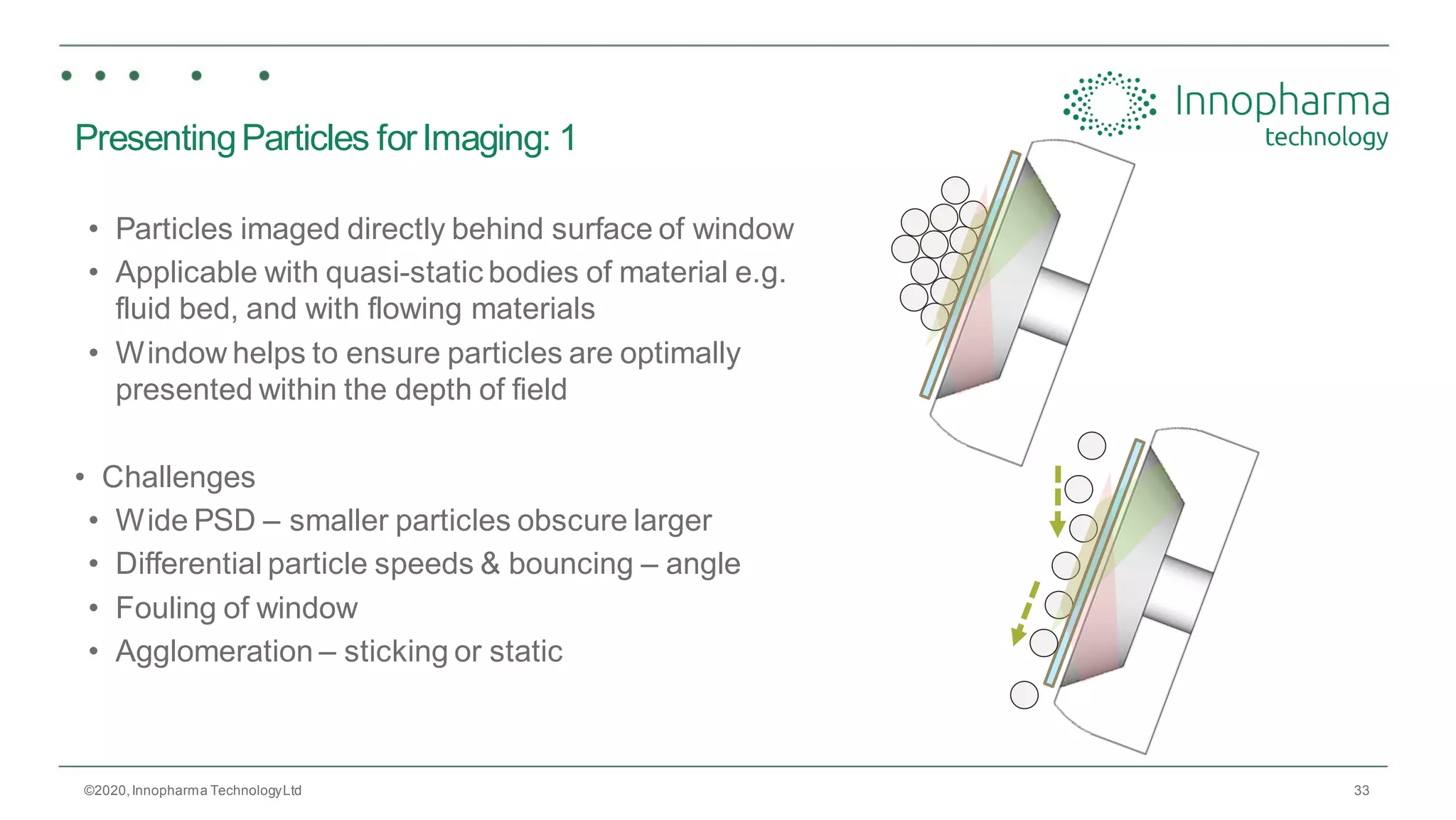
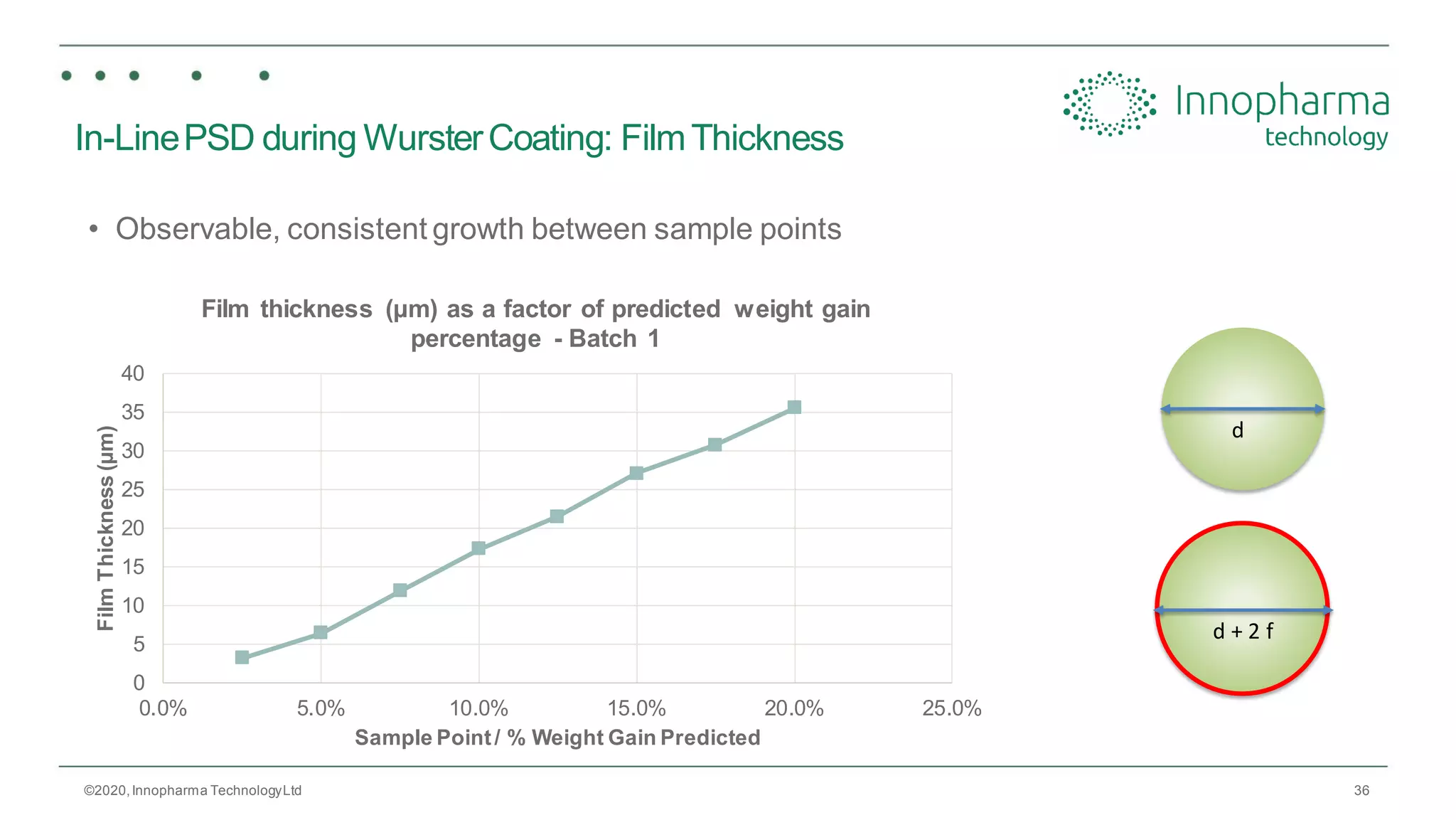
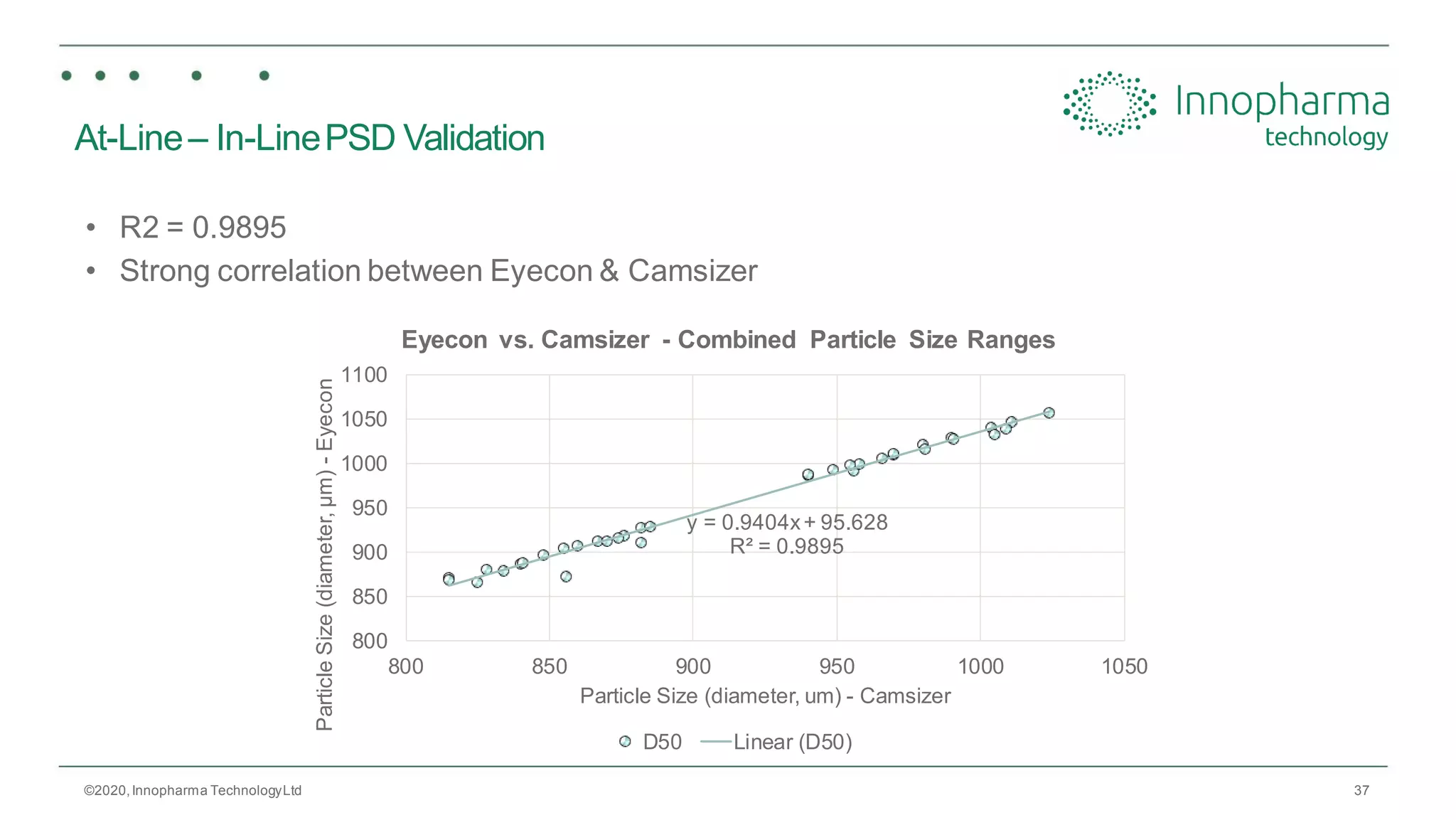
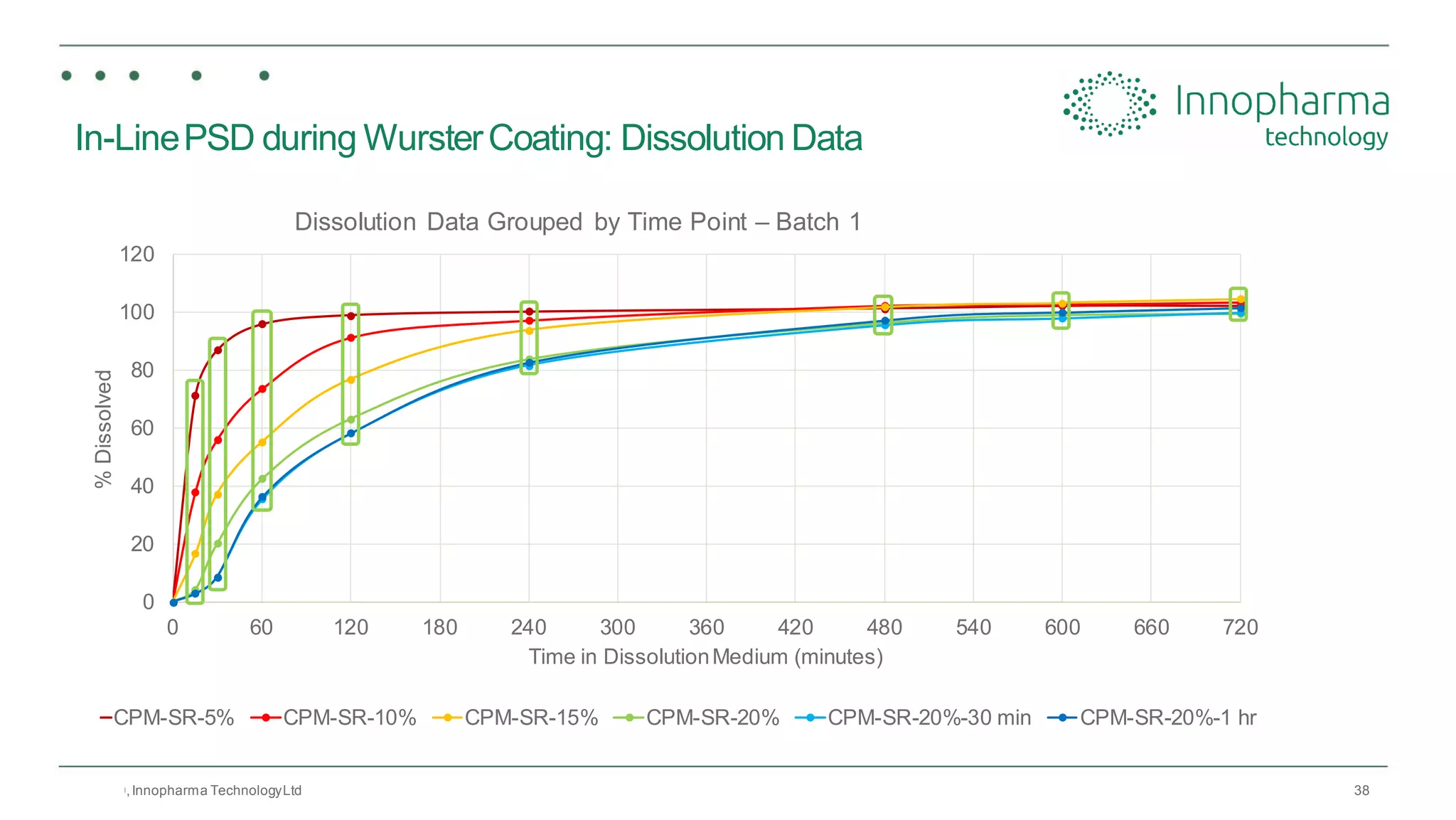
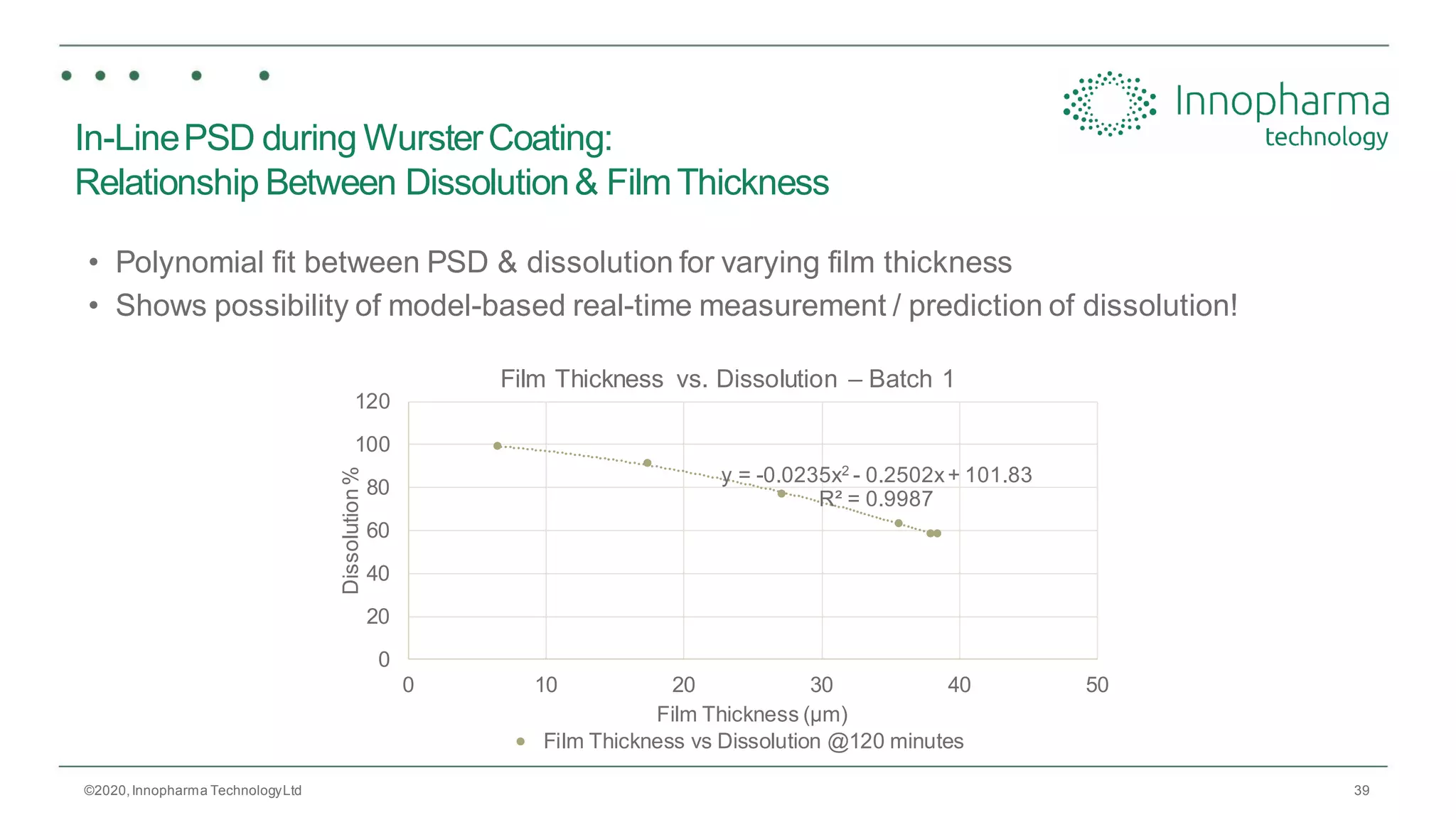
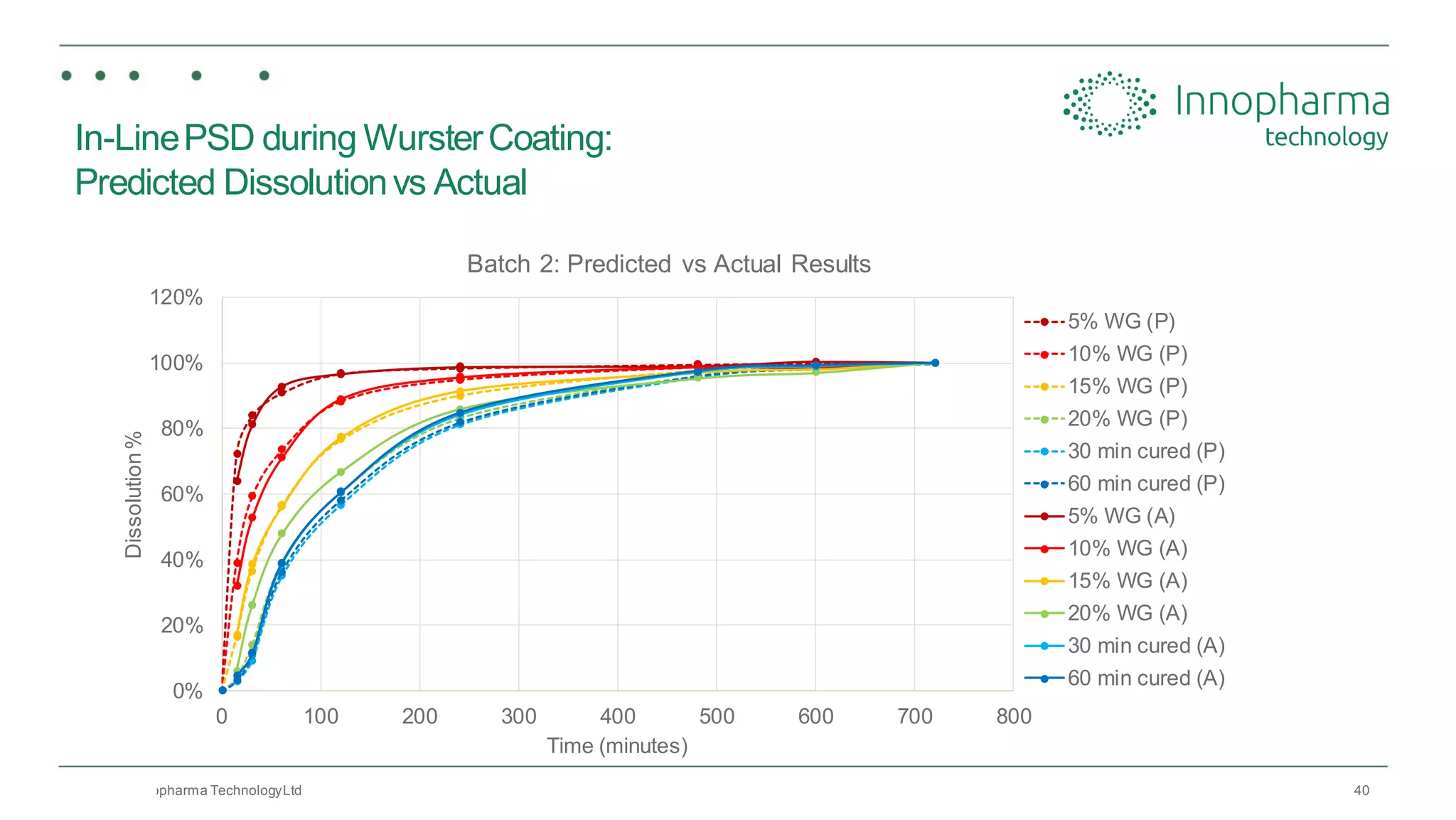
Innopharma Technology provides an overview of its Eyecon2 system, a real-time imaging solution for in-line particle size analysis in pharmaceutical manufacturing processes. The document highlights the system's specifications, applications across various processes like granulation and milling, and its capabilities for enhancing quality control and process efficiency. Additionally, it includes case studies such as Wurster coating, demonstrating its effectiveness in predicting dissolution performance based on particle size measurements.