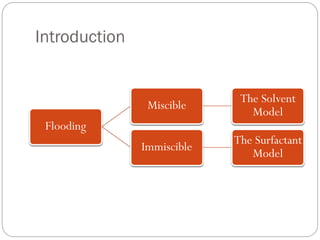
This document provides an overview of the solvent and surfactant models in reservoir simulation. It discusses the objectives and applications of the solvent model, which models miscible displacement processes. It describes the Todd & Longstaff model for representing miscibility and outlines how to treat relative permeability and PVT data. It then discusses the surfactant model, how it models surfactant distribution and its effects on water viscosity, capillary pressure, relative permeability and adsorption.