Embed presentation
Download to read offline
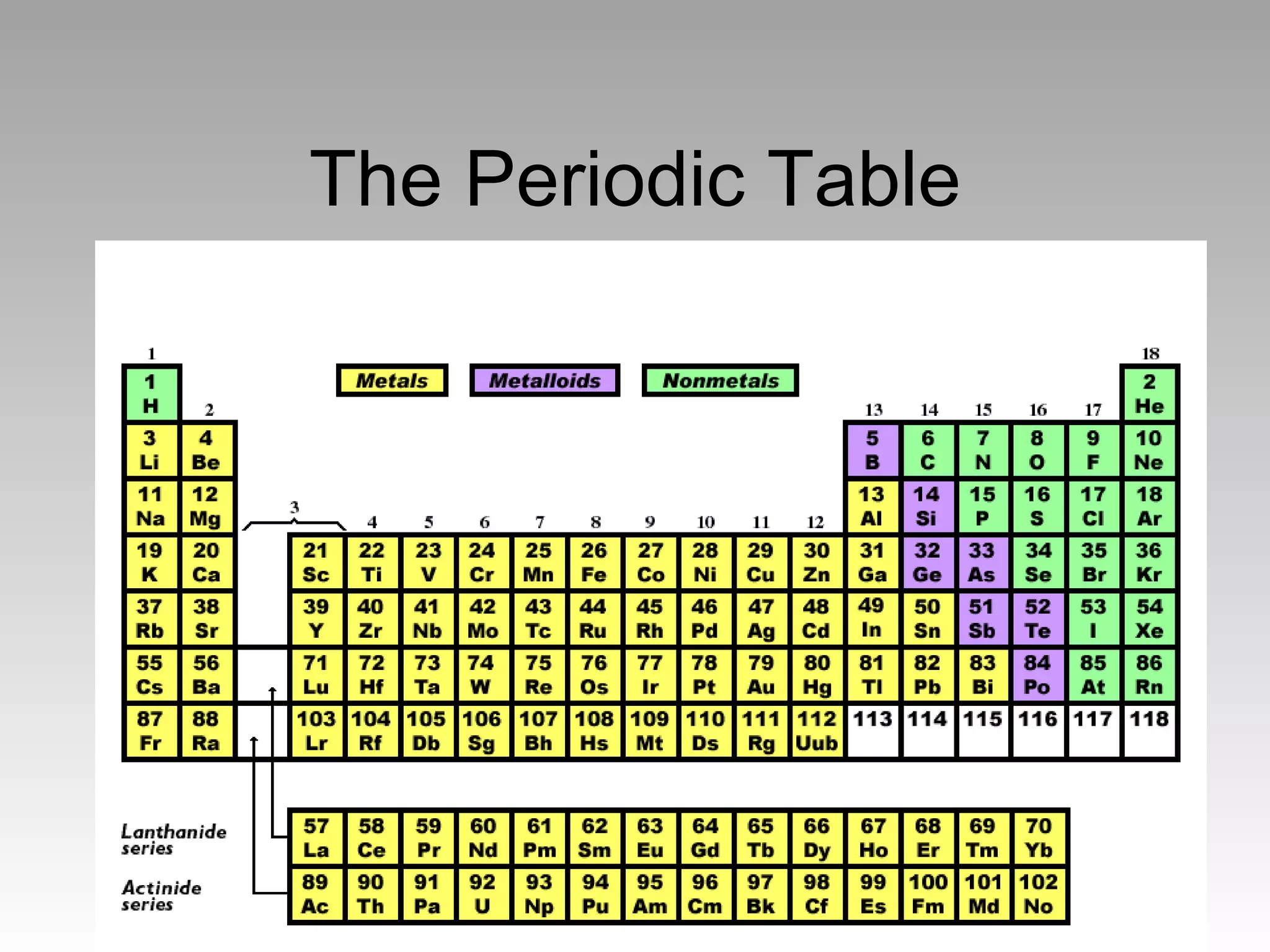

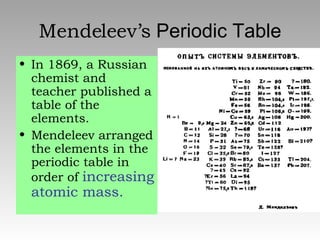

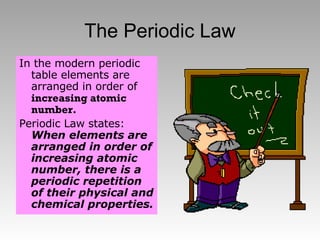
The document summarizes the key developments in the periodic table. It describes how elements are organized in the periodic table with their chemical symbols, atomic numbers, and atomic weights. Early scientists like Dobreiner grouped elements based on their properties. Mendeleev arranged the elements in order of increasing atomic mass. Later, Moseley determined that elements should be ordered by their increasing atomic number. The periodic law states that elements' physical and chemical properties repeat periodically when arranged by atomic number.





