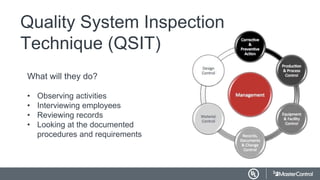
This document provides an overview and guidance for preparing for and responding to a FDA inspection. It discusses having necessary documentation and quality systems in place. When the FDA calls to schedule an inspection, it is important to pull together an inspection team and prepare by reviewing documentation, conducting mock audits, and training employees. During the inspection, the FDA will observe operations, interview staff, and review records. It is important to escort the inspector, answer questions truthfully, and only provide requested documentation. After the inspection, any findings or Form 483 observations must be addressed and corrections provided to the FDA within a specified timeframe.