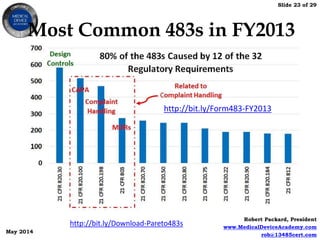
This document provides an overview of FDA inspections of medical device manufacturers. It discusses the FDA's risk-based approach to inspections and types of inspections. Key areas the FDA focuses on include CAPA, management review, internal audits, supplier audits, design controls, complaint handling and adverse event reporting. The document also provides tips on preparing for an inspection, what to expect during an inspection, potential inspection outcomes, and how to respond to FDA observations on Form 483.


![Slide 3 of 29
Robert Packard, President
www.MedicalDeviceAcademy.com
rob@13485cert.com
May 2014
Risk Classification
Class 1 – Low Risks Do Not Require
Pre-Market Notification [i.e. - 510(k)]
Class 2 – Moderate Risks Require 510(k)
& there are “Special Controls”
Class 3 – High Risks & Unknown Risks
Require Pre-Market Approval (PMA)](https://image.slidesharecdn.com/preparingforyournextfdainspectiond3-240415195452-e8e4a0bc/85/Preparing-for-your-next-FDA-Inspection-D3-pptx-3-320.jpg)