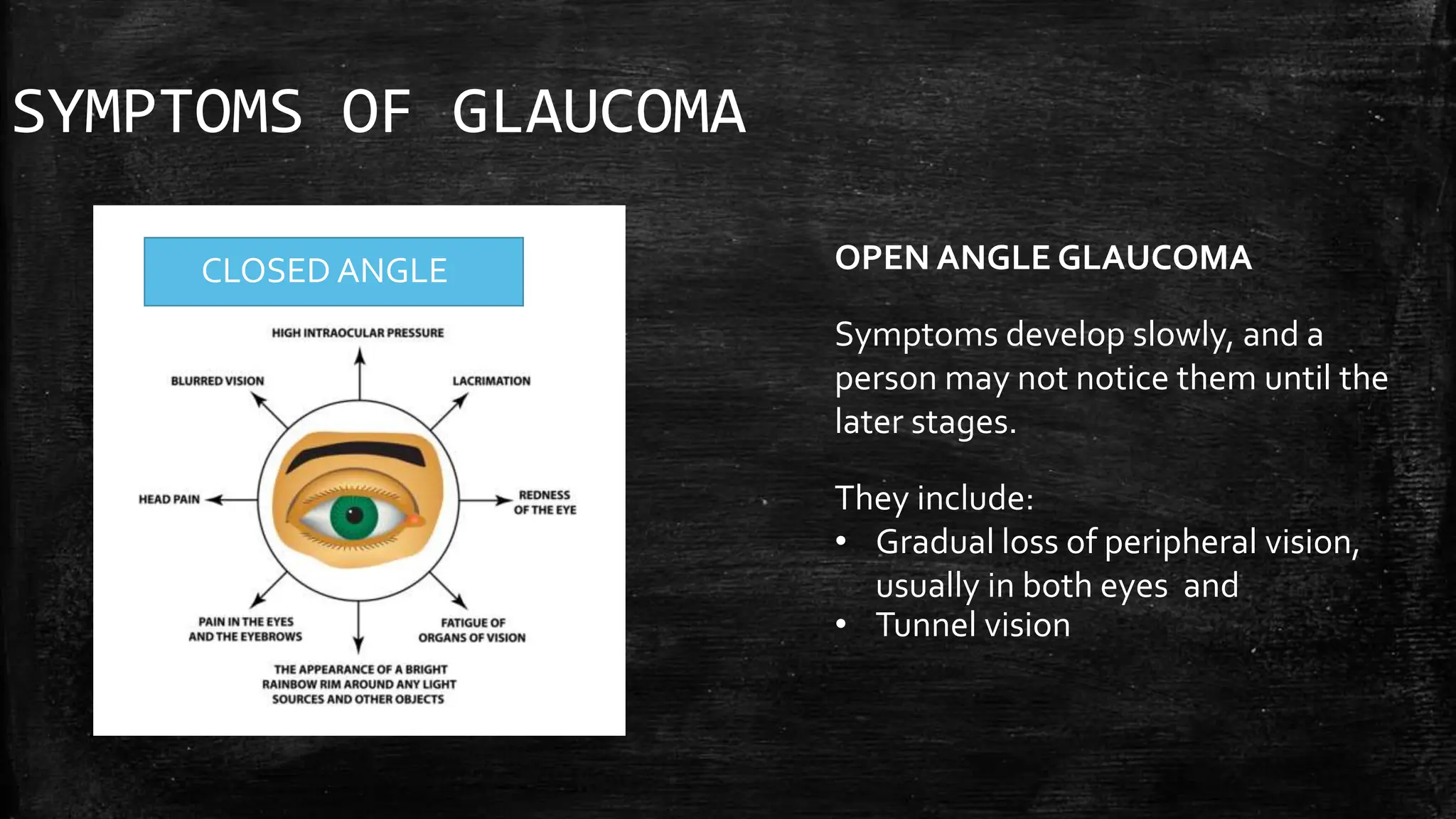
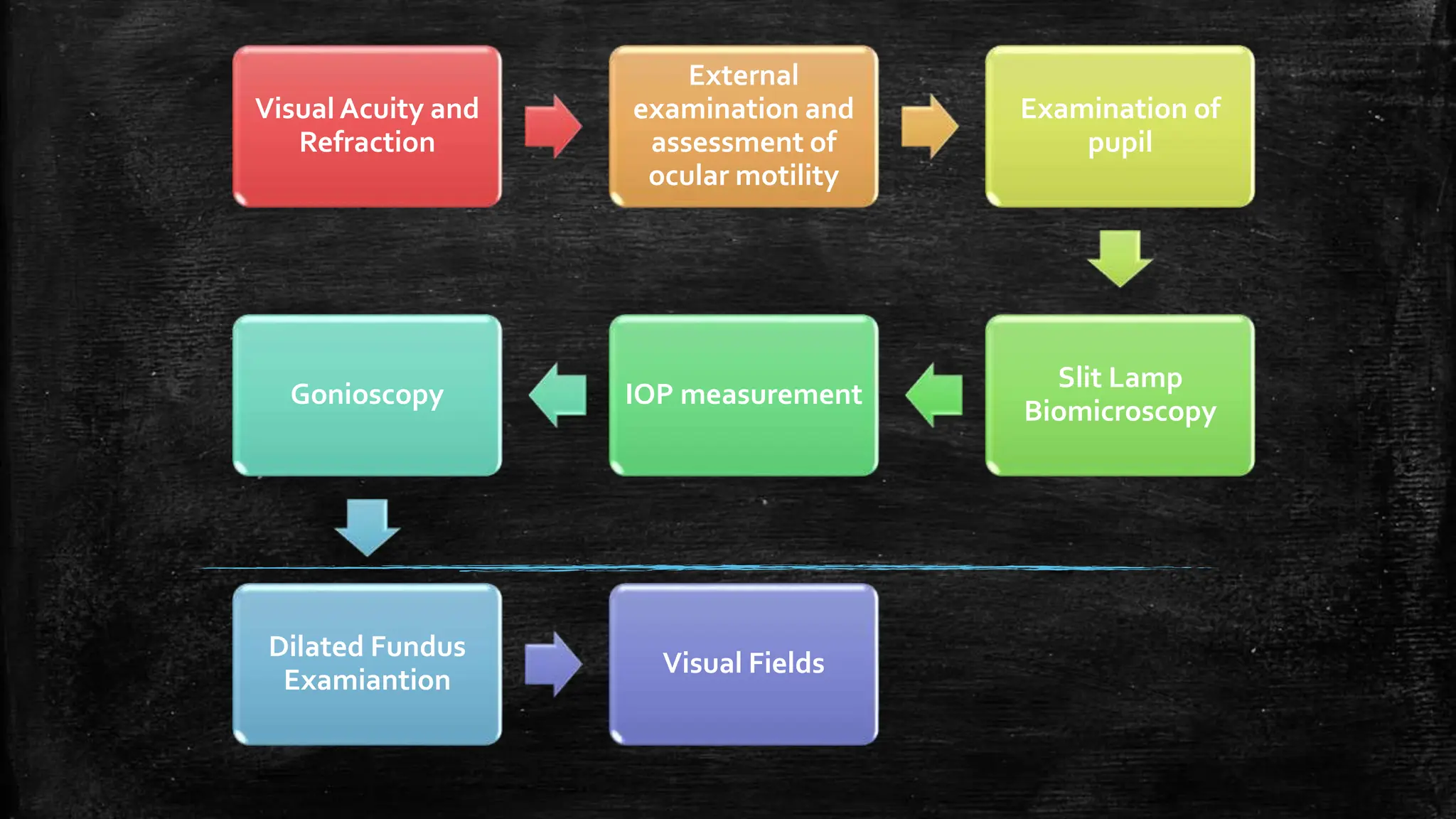
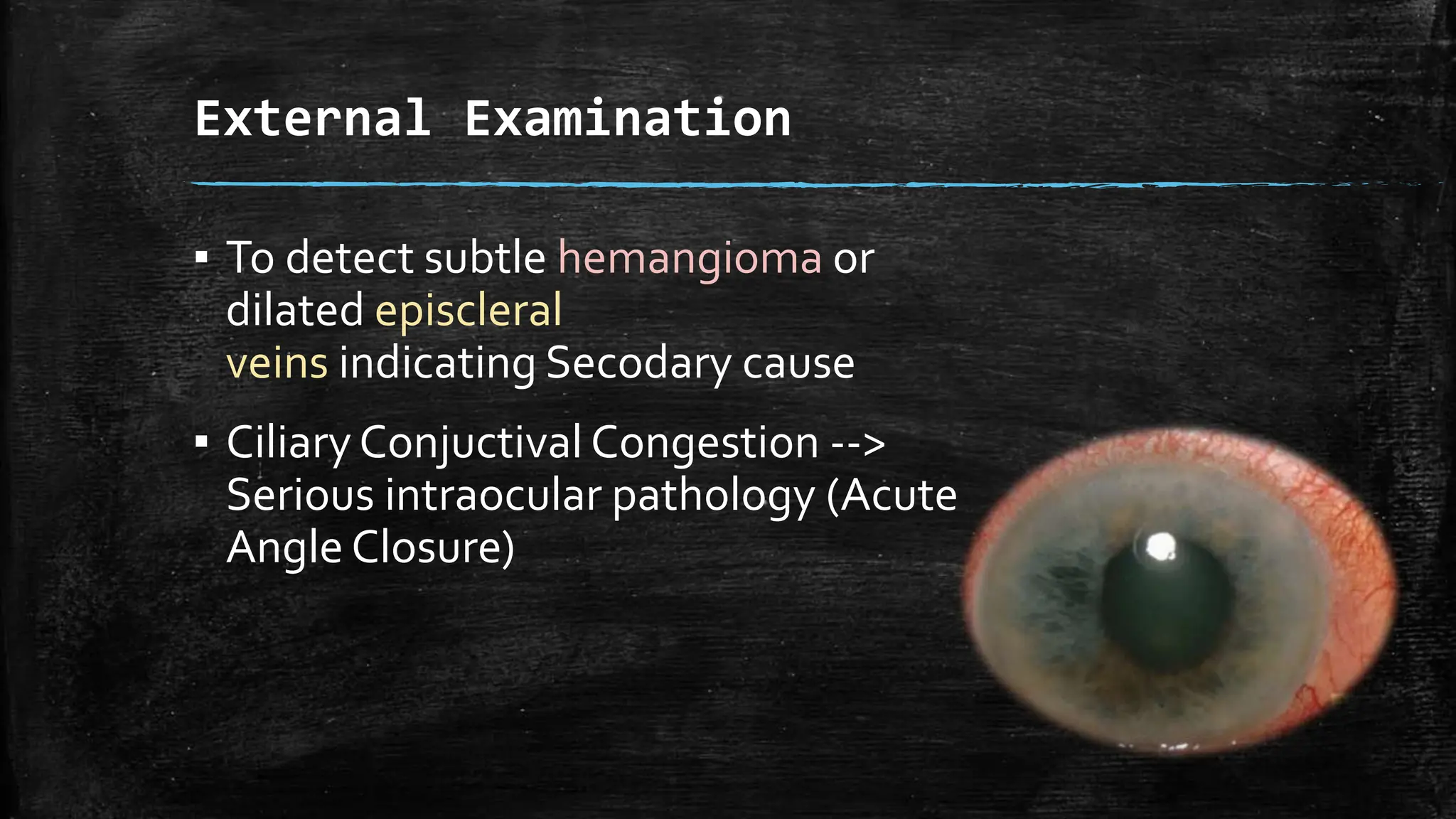
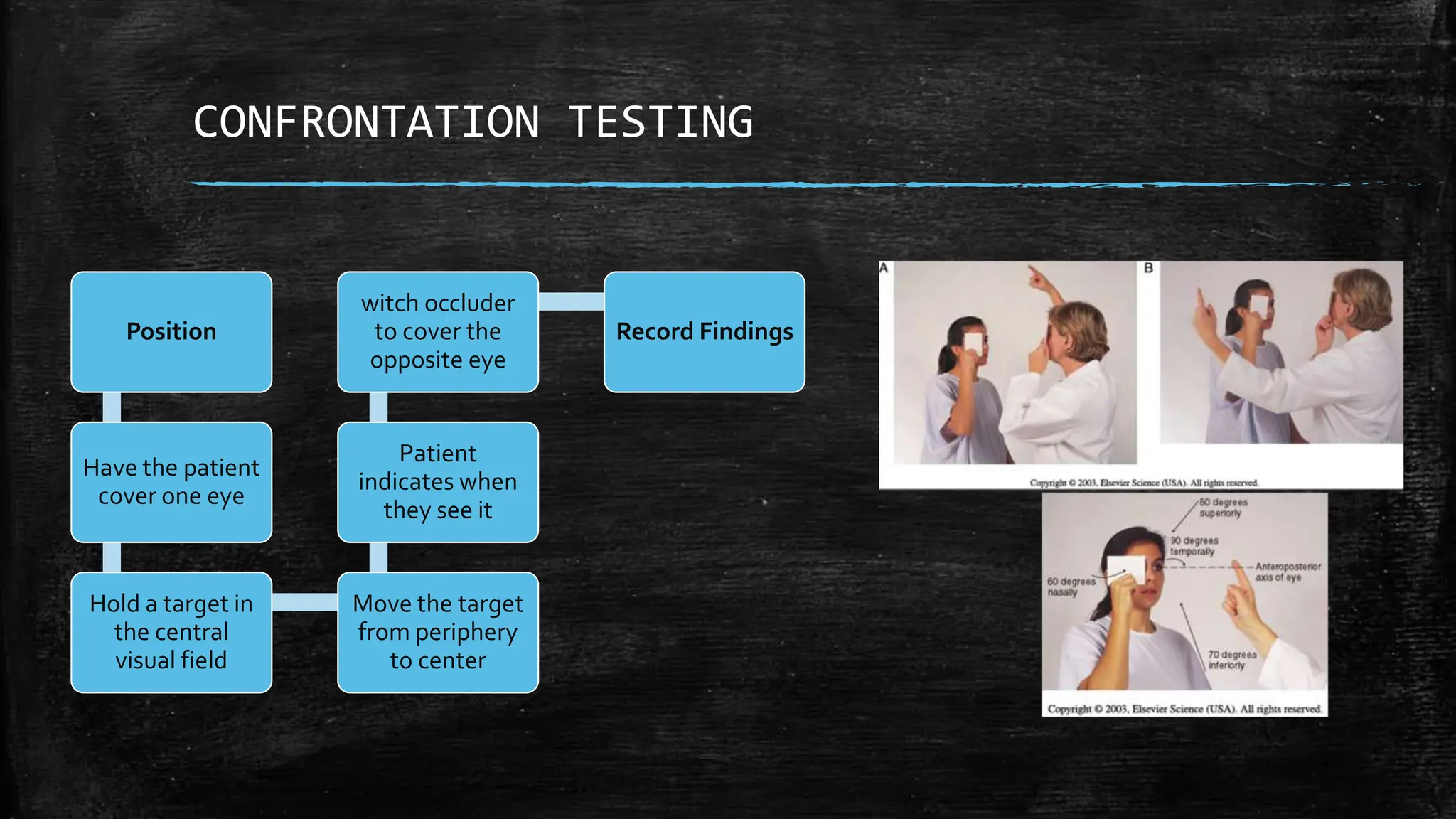
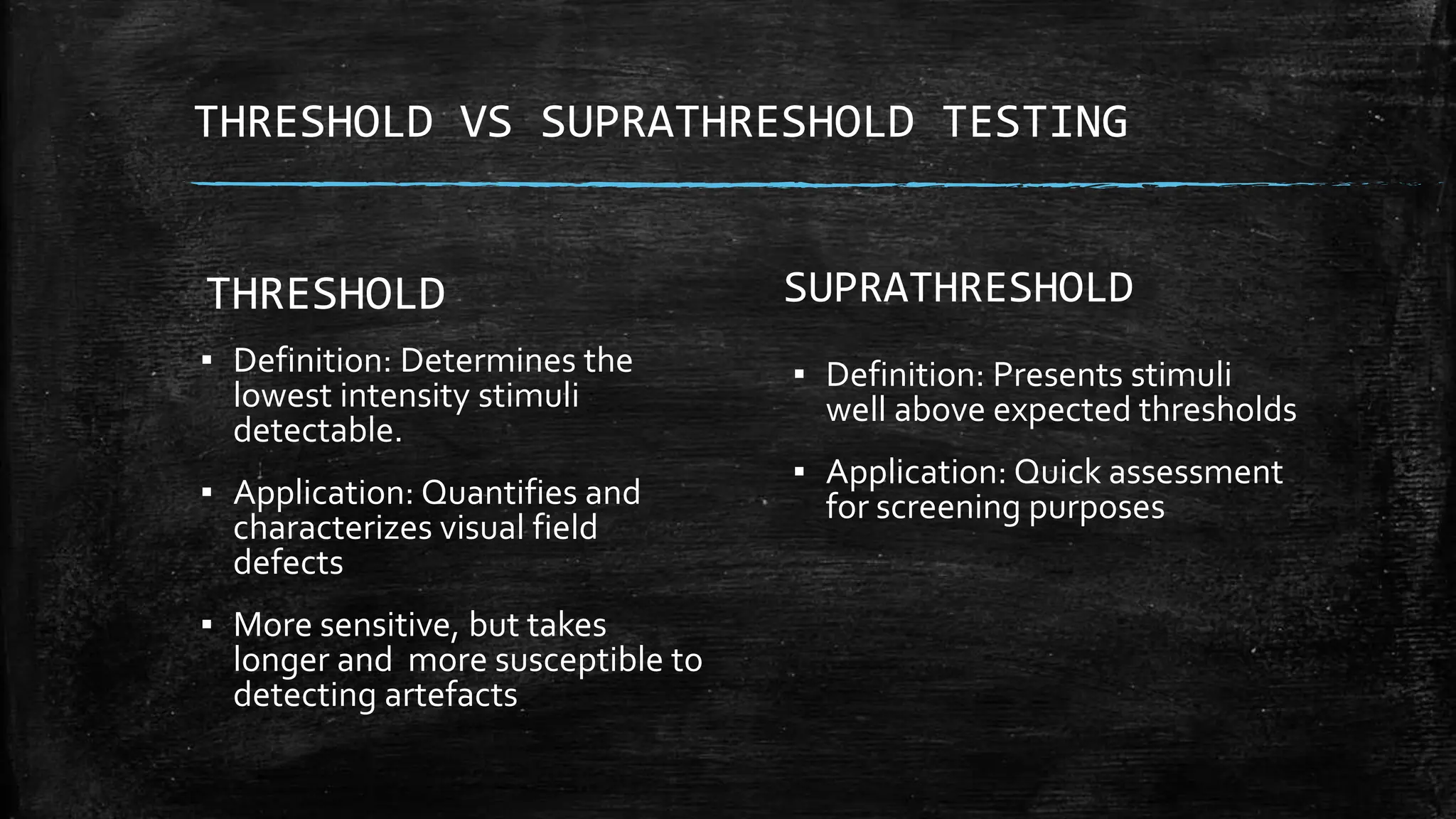
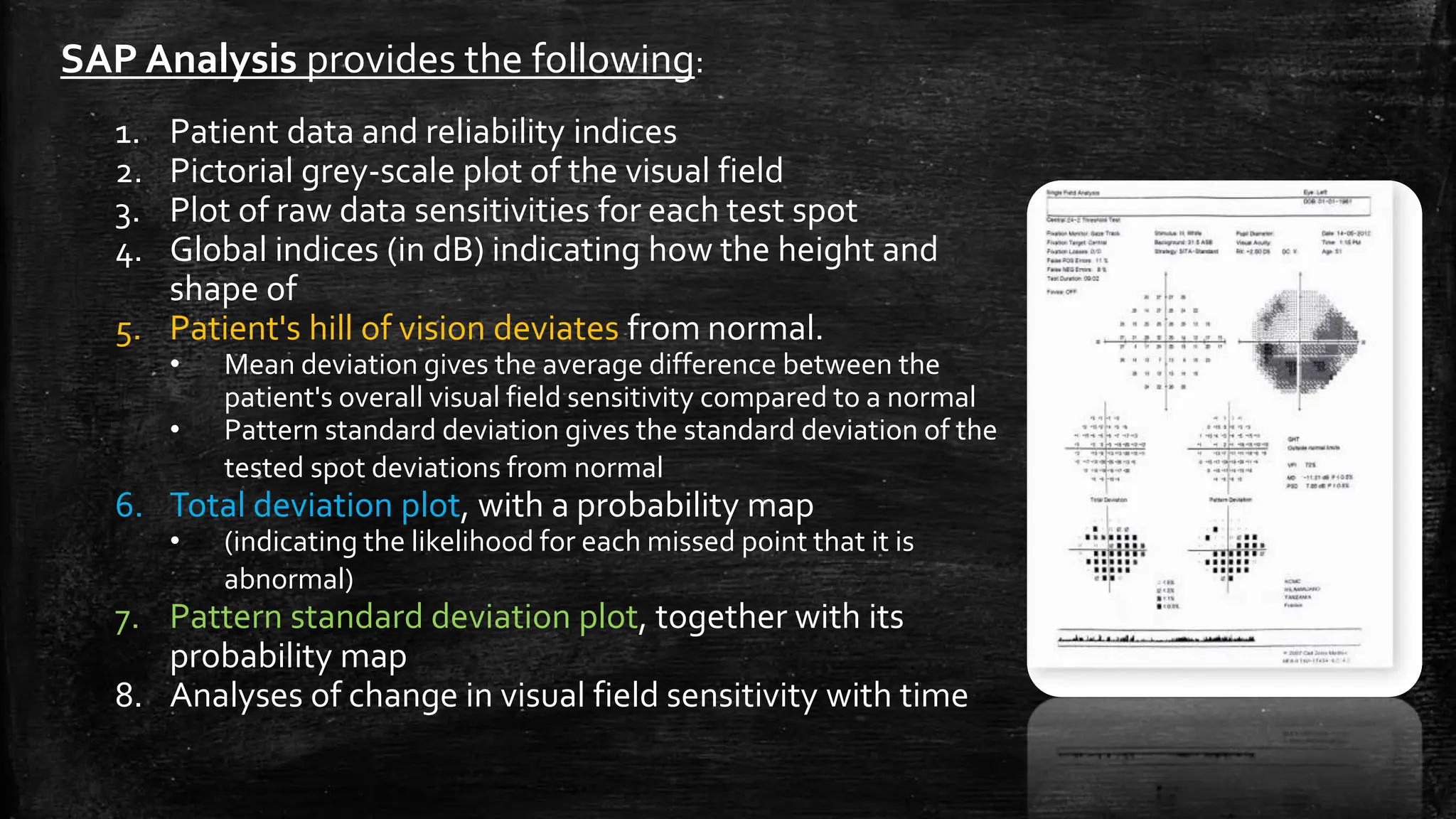
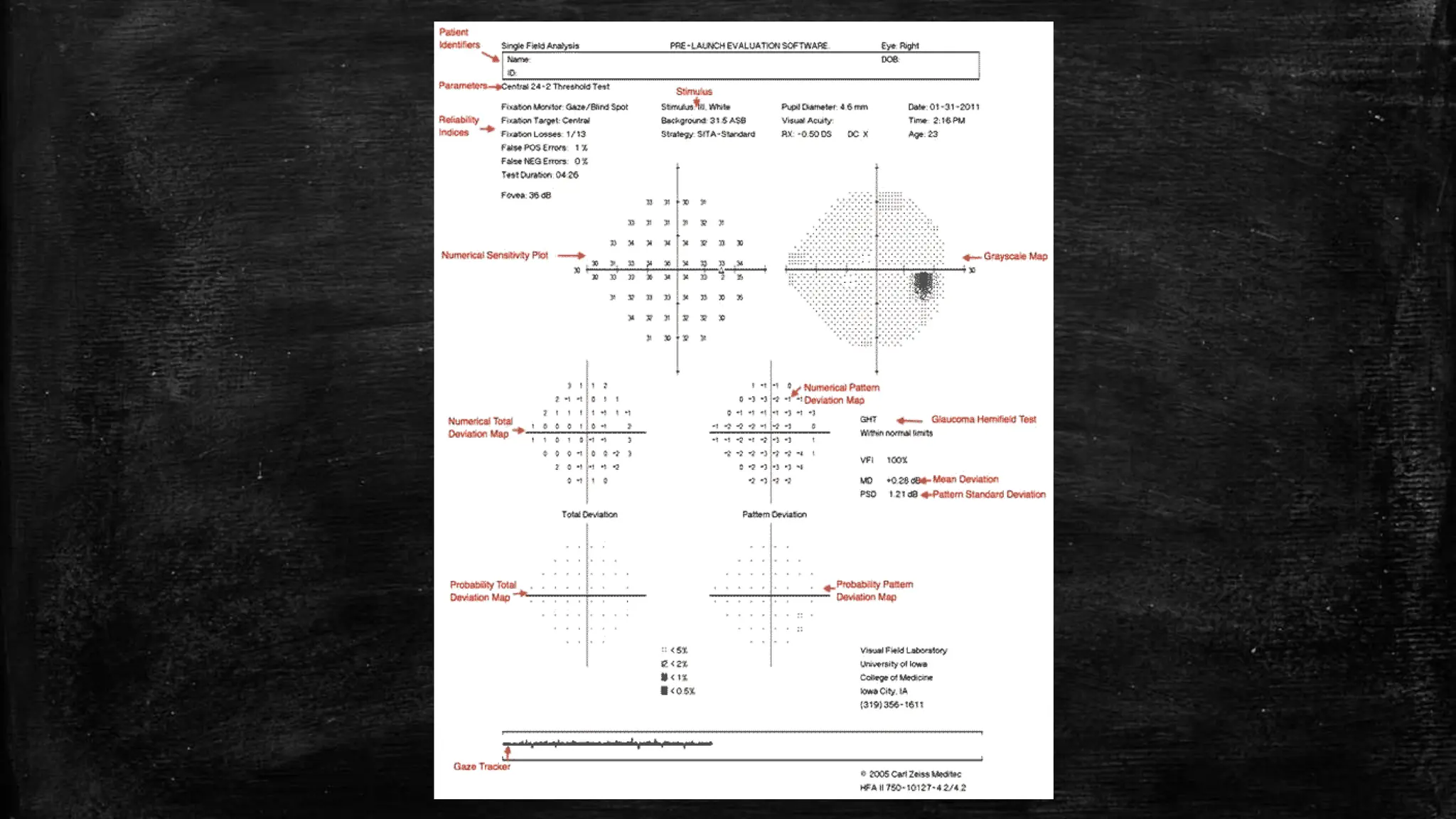
The document provides a comprehensive evaluation of glaucoma, detailing its definition, types, causes, risk factors, symptoms, and methods for examination. Key aspects include the importance of thorough patient history, various diagnostic techniques such as visual acuity tests, intraocular pressure measurement, and visual field testing to assess optic nerve and retina damage. It emphasizes the necessity of identifying signs of glaucoma early to prevent blindness through intervention and management.