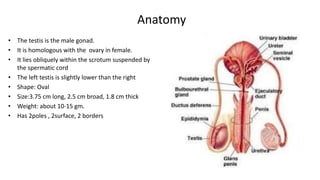
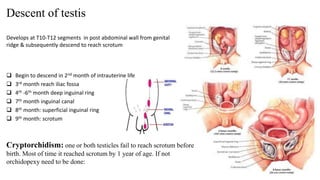
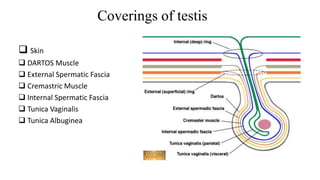
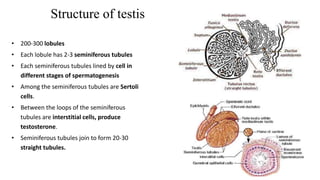
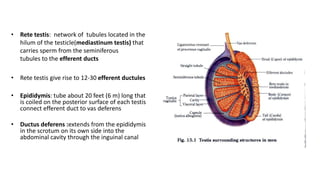
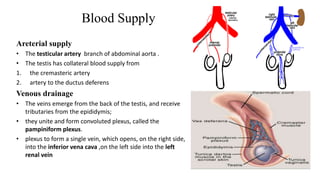
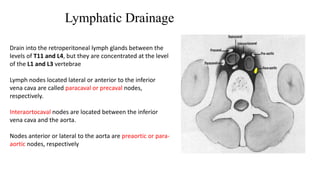
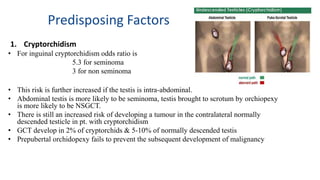
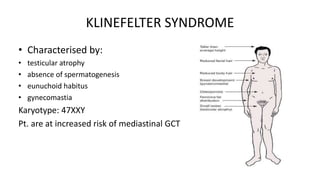
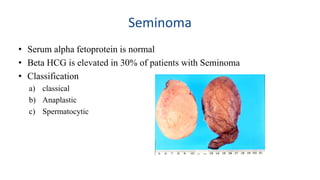
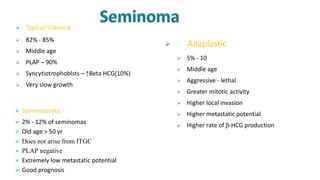
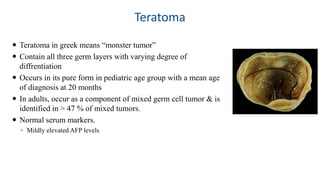
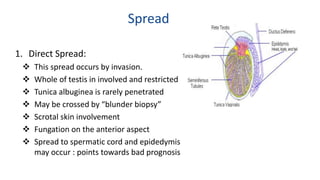
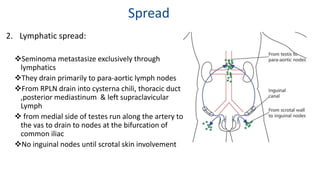
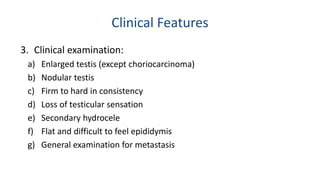
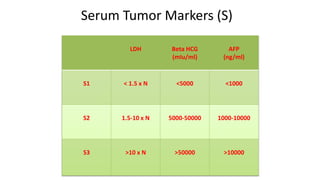
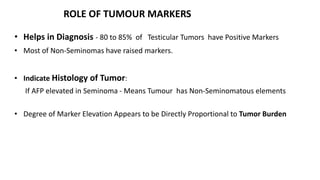
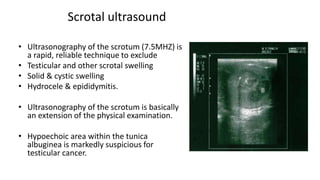
Testicular cancers are rare but highly treatable neoplasms predominantly affecting young males, constituting about 1% of all male cancers. The document outlines the anatomy, epidemiology, predisposing factors, and classifications of testicular tumors, emphasizing the importance of tumor markers in diagnosis and treatment. It discusses different types of germ cell tumors and secondary tumors, highlighting key clinical features and implications for management.