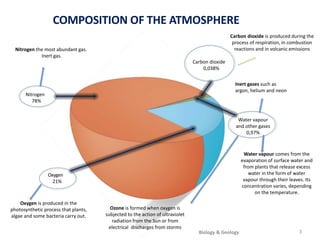
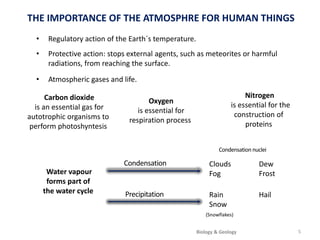
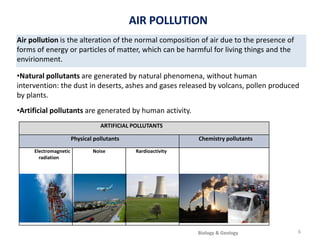
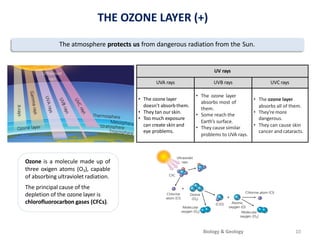
The atmosphere is the gaseous layer surrounding the Earth. It is composed primarily of nitrogen (78%) and oxygen (21%), along with smaller amounts of other gases like carbon dioxide and water vapor. The atmosphere regulates the Earth's temperature and protects the surface from harmful radiation and meteorites. It also provides essential gases for life, such as oxygen for respiration and carbon dioxide for photosynthesis. However, human activities like burning fossil fuels have increased greenhouse gases and depleted the ozone layer, contributing to issues like global warming, acid rain, and increased UV radiation at the surface.