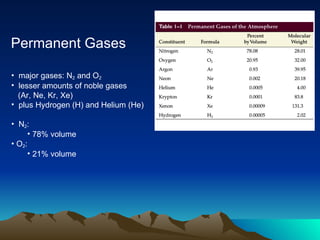
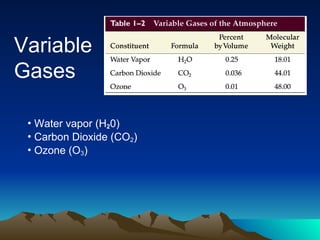
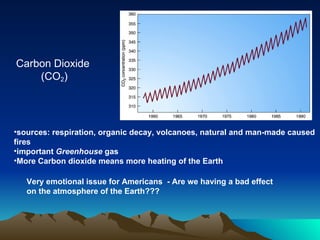
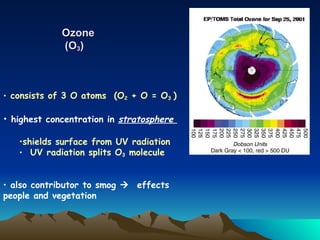
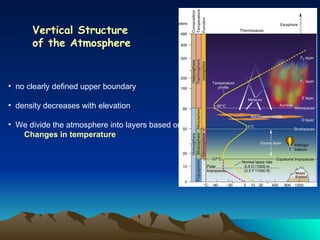
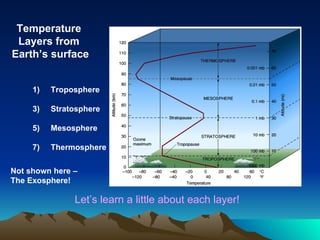
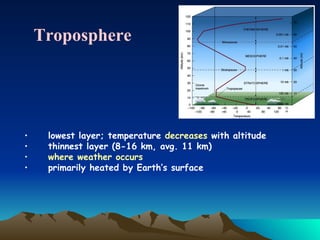
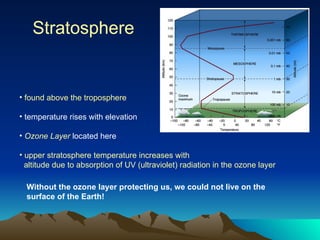
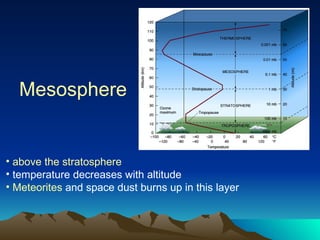
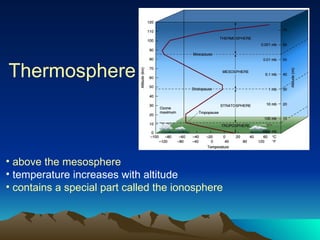
The document summarizes the composition and structure of Earth's atmosphere. It is composed primarily of nitrogen (78%) and oxygen (21%), as well as smaller amounts of other gases like carbon dioxide, water vapor, and ozone. The atmosphere does not have a clear upper boundary and becomes less dense with increasing altitude due to decreasing air pressure. It can be divided into layers based on changes in temperature, density, and other factors. These layers include the troposphere, stratosphere, mesosphere, and thermosphere.