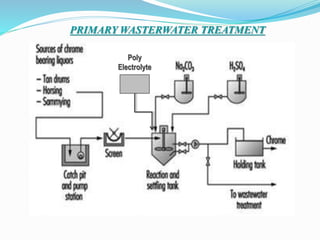
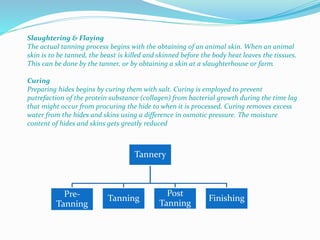
This document provides information about chrome tanning training done at Super Tannery India Ltd. It discusses the company background and various leather products produced. It then describes the multi-step chrome tanning process, including soaking, liming, pickling, tanning, post-tanning operations, and finishing. Quality tests are presented to check physical and chemical properties of the finished leather. Wastewater generation and treatment during tanning are also summarized. Purity tests done to check chromium oxide content and treat effluent are mentioned at the end.










![Chrome-tanned leather, invented in 1858, is tanned
using chromium sulfate and other salts of chromium. It is
more supple and pliable than vegetable-tanned leather and
does not discolor or lose shape as drastically in water as
vegetable-tanned. It is also known as wet-blue for its color
derived from the chromium. More esoteric colors are
possible using chrome tanning.
Chromium(III)sulfate([Cr(H2O)6]2(SO4)3) has long been
regarded as the most efficient and effective tanning
agent. Chromium(III) compounds of the sort used in
tanning are significantly less toxic than hexavalent
chromium.
CHROME TANNING](https://image.slidesharecdn.com/d3a7c37e-fd8c-4da8-854a-ffed9a4c1e7c-150518143814-lva1-app6892/85/summer-training-ppt_2_1-13-320.jpg)