Herbal drug technology Unit IV PCI syllabus semester VI
- 1. QUALITY EVALUATION OF HERBAL DRUGS Standardization & Quality Evaluation of Herbal drugs
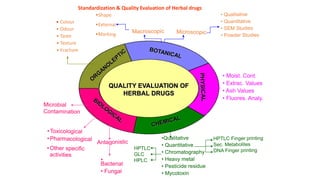
- 3. WHO Guidelines for the Evaluation of herbal drugs: 1. Foreign matter 2. Organoleptic evaluation 3. Physicochemical evaluation 4. Moisture content 5. Determination of toxic content 6. Microbial contamination 7. Pesticide residue 8. Radioactive contamination and heavy metals.
- 4. • ICH of regulatory requirements was pioneered by the EC, Europe, in 1980s, as the EC, Europe moved towards the development of a single market of pharmaceuticals. • The success achieved in Europe demonstrated that harmonization was feasible. • At the same time there was discussion between Europe, Japan and US on possibilities for harmonization. • It was, however, at the Paris, in 1989, that specific plan for action began to materialize. • The birth of ICH took place at a meeting in April 1990, hosted by EFPIA in Brussels. • At the first ICH steering committee meeting the term of reference were agreed and it was decided that the topics selected for harmonization would be divided into safety, quality and efficacy to reflect the three criteria which are the basis for approving and authorizing new medicinal products. Role of ICH in Evaluation of Herbal drugs:
- 5. Role of ICH in Evaluation of Herbal drugs: ICH guidelines are divided into four types: QSEM 1. Quality guidelines, 2. Safety guidelines, 3. Efficacy guidelines and 4. Multidisciplinary guidelines
- 6. 1. Quality guidelines include stability, analytical validation and quality of biotechnological product, GMP, Pharmaceutical development etc. 2. Safety guidelines of ICH uncover the potential risk of carcinogenicity, genotoxicity etc. It also includes pharmacological studies, immunotoxicity studies, photo safety and non- clinical pediatric safety studies. 3. Efficacy guidelines mainly covers the design, conduct, safety and reporting of clinical trials. 4. Multidisciplinary guidelines for gene therapy, mutagenic impurities, bioanalytical method validation etc. Role of ICH in Evaluation of Herbal drugs:
- 7. Three major steps to assure quality in herbal drugs as per WHO/ICH guidelines are: 1. Identification /ensuring genuinely of herbal drugs 2. Purity/ensure safety of herbal drugs 3. Ensure Efficacy of herbal drugs
- 8. 1. Assessment of quality of herbal drugs as per WHO/ICH guidelines: [GACP------- Taxonomic identification------- Morpho-anatomical, Phytochemical, genome coding etc.] Pharmaceutical Assessment Crude plant material Plant preparation Finished product Stability 2. Assessment of safety Toxicological studies Documentation of safety based experience 3. Assessment of Efficacy: (Pharmacological/therapeutic and clinical effect) Combination Products
- 9. WHO/ICH guidelines for Stability Testing of Herbal Drugs: • According to modern medicine system, stability is the capacity of a specific formulation in a particular container/closure system to remain within its prescribed limits like physical, chemical, microbiological, toxicological, therapeutic specifications. • Stability is define as “the capacity of a drug to remain within established specifications limits to maintain its identity, strength, quality and purity throughout the reset or expiration dating periods” • The shelf life of a product can be defined as, “the time duration up to which it is expected to retain 90 % of its active ingredients (label claim) when stored in recommended condition”
- 10. Stability testing Advantages: To determine shelf life and storage condition Selection of suitable formulation, excipients and container closure system for a product Wellbeing of patient
- 11. Stability Studies • Stability is the capacity of a drug substance or product to remain within the established specifications which maintain its identity, strength, quality, and purity throughout the retest or expiration dating period. • Stability study aims to determine the shelf-life and the time period of storage at a specified condition within which the drug product meets its established specifications. • Stability influences the quality, safety and efficacy of a drug product. • A drug product of insufficient stability can change the physical (hardness, dissolution rate, phase separation, etc.) and chemical characteristics (formation of high risk decomposition substances). • Stability evaluation of drug substance or product is the key to drug quality as it determines the efficacy of any drug or dosage form. • Stability testing proves that the quality of a drug substance or product changes with time under the influence of various environmental conditions such as temperature, relative humidity, light, etc. • Stability study involves a series of tests to obtain an assurance of drug product stability, namely maintenance of the drug product packed in it specified packaging material and stored in the established storage condition within the determined time period.
- 12. Importance of Stability testing: It evaluates the efficacy of a drug. Stability studies are used to develop suitable packaging information for quality, strength, purity & integrity of product during its shelf life. It is used for determination of the shelf life. Stress testing: Stress testing help to identify the degradation product, which can help to establish the degradation pathway. Stress tests are usually considered unnecessary for herbal drug & its preparation. 1. For herbal drugs and herbal drug preparations, a testing under accelerated or intermediate conditions may be omitted. This should apply to finished products as well, because it is known that most products fail at 30°C/65 per cent relative humidity (RH) and at 40°C/75 per cent RH in particular. 2. If intermediate conditions are tested, the three-month time-point is omitted (that is, 0, 6, 9 and 12 months). In some cases of combination products, it is hardly possible to provide the required two batches of each extract at the same time due to different harvesting times.
- 13. Selection of batches: Long term testing is to be provided with at least two batches of the drug substance and three batches of drug product. In some cases of combination products, it is hardly possible to provide the required two batches of each extract at the same time due to different harvesting times. This should be taken into consideration when planning the schedule for stability study. Predictable changes in Herbal medicinal Product: Following predictable changes may occurs in herbal medicinal product during storage and in shelf life determination: Hydrolysis, Oxidation, Racemization, Geometric isomerization, Temperature, Moisture and Light Hydrolysis: Reaction with water takes place results in degradation of product. Oxidation: Due to addition of electro negative atom, Removal of electro positive atom, radicals formation results in decomposition of natural products. Racemization: Racemization is the process in which one enantiomer of a compound, such as an L- amino acid, converts to the other enantiomer. The compound then alternates between each form while the ratio between the (+) and (–) groups approaches 1:1, at which point it becomes optically inactive. Geometric isomerization: Products can be change in trans or cis form. One form may be more therapeutically active. Polymerization: There is combination of two or more identical molecule to form much larger & more complex molecule.
- 14. Temperature: The rate of most chemical increase with increase in temperature. So that “Tropical” area must be taken in consideration during preparation of the formula of the herbal substance. Moisture: Moisture absorbed on to the surface of solid drug will often increase the rate of decomposition, if it is susceptible to the hydrolysis. Light: Many type of chemical reaction induced by exposure to light of high energy. Autoxidation of volatile oil / fixed oil takes place and substance becomes colored.
- 43. Sr. no Type of IPR Law Protection given Application/example Duration in years 01 Patents IP Act 1970 Discoveries and inventions Drugs, medicine, scientific discovery 20 years after filing, cant be renewed 02 Trademarks Trade Mark Act 1999 Phrases, symbols and designs Business branding 10 years duration can be renewed for again 10 years 03 Copy right Copyright Act, 1957 Musical, literacy and artistic work Books, drama, literature Lifetime of author or artist and 60 years after death 04 Industrial design Design Act 2000 Design of machine, equipment etc Manufacture of equipment, industries Initial 10 years can be extended to 5 more years 05 Plants The plant variety protection Act 1970 Plant variety, farmers right, trees Farmer 15 years from date of registration 06 Geographical goods The geographical Indication of Goods (Registration and Protection Act, 1999) Certain goods Basmati Rice, Banarasi silk, Madhubani painting Initial 10 years can be extended for another 10 years Intellectual Property Rights
- 46. A patent may be granted only for an invention in respect of which the following conditions are satisfied: 1.The invention is new 2.Involves an inventive step 3.Is capable of industrial application or utility.
- 47. What is ZOLGENSMA? ZOLGENSMA is a prescription gene therapy used to treat children less than 2 years old with spinal muscular atrophy (SMA). ZOLGENSMA is given as a one-time infusion into a vein. ZOLGENSMA was not evaluated in patients with advanced SMA. •ZOLGENSMA can increase liver enzyme levels and cause acute serious liver injury or acute liver failure. •Patients will receive an oral corticosteroid before and after infusion with ZOLGENSMA and will undergo regular blood tests to monitor liver function. •Contact the patient’s doctor immediately if the patient’s skin and/or whites of the eyes appear yellowish, if the patient misses a dose of corticosteroid or vomits it up, or if the patient experiences a decrease in alertness. The world’s most expensive drug Zolgensma (generic name: onasemnogene abeparvovec-xioi) is a life-saving gene therapy approved in May 2019 to treat paediatric Spinal Muscular Atrophy (SMA). Zolgensma's total cost is $2.125 million, according to Novartis, the manufacturer. It's considered the world's most expensive single- dose drug. (That is 16,50,95,925.00 Indian Rupee)
- 48. A) Patent and Natural Products: Patents comes under IP, patents may provide the strongest IP protection, but to get the IP protection for natural or herbal medicine is very difficult. To qualify for a patent, an invention must be novel, useful. Most natural or herbal medicine have been used or practices from more than hundreds of years, thus their novelty comes into question. Also, the product being patented cannot have been publically used or known. So most natural products come under prior art.
- 49. According to Second Amendment of Patent Act 2002 following things are not patentable: Plants, animals in whole or any part Seed or varieties of plant Biological processes for production of plants and animals Microorganisms can be claimed for patent provided if they are not only discovery of organism existing in nature Methods for rendering plants free of diseases or to increase their economic value will be patentable.
- 50. Indian Patent Act 1. Patent act 1970 Most significant act in the legislature of protection of IPR Act came into force on 20th 1972 As per this act the major prerequisites for patenting in India are - A new invention - Should be new and non obvious with respect to the prior art - It must be useful -Not previously in use in India
- 51. Invention, as per the act may be defined as any new and useful Art, process, method of manufacture Machine, apparatus or other article Substances produced by manufacture, including any new and useful improvements of any of them Indian Patent Act
- 52. Some examples in Pharmacy, which are patentable are New process of manufacture (that is called process patent) New Chemical Entities (NCE) (product patent) Indian Patent Act
- 53. Following are non patentable in India Discoveries Method of detection, diagnosis or treatment of diseases Analytical methods Methods of agriculture/cultivation Animals, plants and biological methods for rearing and growing them Indian Patent Act
- 60. Rights of a Patentee Right to exploit the patent The patentee has a right to prevent 3rd parties, from exploiting the patented invention Right to grant license The patentee has a power to assign rights or grant license Right to surrender The patentee is given the right to surrender the patent by giving notice in prescribed manner to the controller
- 61. Protection of Plant Varieties and Farmer’s Right Act, 2001 (PPVFR Act) Breeder means a person or group of persons or a farmer or group of farmers or any institution which has bred, evolved or developed any variety. Protection to all types of plants except microorganism was given. For trees and vines 18 years protection is given. For all other plants and extant varieties 15 years protection was given as per section 5 of the seed act 1966.
- 62. Bioprospecting and Biopiracy The process of finding new chemicals or drugs from various natural resources is called as bioprospecting. It is also define as the process of discovery and development of new molecules from different biological resources. More than 200 companies and research organizations worldwide are screening plant and animals compounds for possible new drug candidates. Because of high throughput technologies, screening of new chemical has become easy and many candidates can be screened within short time. During 1980 to 2010, one third of all small molecule chemical entities approved by the U.S FDA were directly or indirectly derived from natural products.
- 64. Biopiracy is a sort of theft. Biopiracy mainly focuses on the use of biological resources and /or knowledge of indigenous tribes or communities without giving them benefits. Biopiracy is an injustice relating to patent Incident related to biopiracy are not necessarily related with money but also pride of country and people. Various international organizations like the Secretariant of the United Nations Convention on Biological Diversity (CBD), the World Intellectual Property Organisation (WIPO) and World Trade Organisation (WTO) are associated with this issue.
- 65. Parameters Biopiracy Bioprospecting Definition The unethical or illegal appropriation and exploitation of indigenous knowledge, traditional practices, or biological resources. The systematic search, collection, and analysis of biological resources for commercial or scientific purposes, with proper consent. Nature Unethical and illegal Legal and ethical (when conducted responsibly) Consent Unauthorized use without obtaining proper consent from indigenous communities or countries. Requires obtaining informed consent from indigenous communities or countries. Benefit sharing Lack of fair compensation or benefits provided to the communities or countries possessing the knowledge or resources. Emphasizes fair and equitable sharing of benefits with indigenous communities or countries. Patents and rights Unauthorized patenting and commercialization of traditional knowledge, genetic resources, or biological samples. Respect for intellectual property rights and sharing of benefits with the source communities. Purpose Exploitative, with the primary focus on maximizing profits for the entity carrying out the exploitation. Aimed at scientific research, development of new products, and fair economic development for the communities involved. Perception Criticized for exploiting the intellectual property of indigenous peoples and local communities. Viewed as a potential tool for sustainable development and conservation of biological diversity when conducted responsibly.
- 66. Biopiracy examples 1.Neem Tree: In the 1990s, a US-based corporation patented the use of neem tree extracts for pesticidal and pharmaceutical purposes without providing proper compensation to the indigenous communities in India and Africa where the neem tree has been traditionally used for centuries. 2.Hoodia Plant: Hoodia, a succulent plant found in Southern Africa, gained attention for its potential appetite suppressant properties. A British company patented the active ingredient without proper authorization or benefit sharing with the indigenous San people who had traditional knowledge of the plant.
- 67. Bioprospecting: 1.Bioactive Compounds from Marine Organisms: Scientists and pharmaceutical companies are engaging in bioprospecting activities in oceans and seas to discover and develop new drugs from marine organisms. Examples include the discovery of potential anticancer compounds from sponges or antibiotic substances from bacteria found in deep-sea hydrothermal vents. 2.Traditional Medicinal Knowledge: Researchers and pharmaceutical companies are collaborating with indigenous communities to document and study their traditional medicinal knowledge. This includes the identification of active compounds from plants used in traditional medicine, leading to the development of new drugs or natural products.
- 76. Parameters Farmers' Rights Breeders' Rights Definition Customary rights of farmers to save, use, exchange, and sell seeds Intellectual property rights for new plant varieties Focus Preservation of agricultural biodiversity Incentivizing plant breeding and innovation Rights - Save, use, exchange, and sell farm-saved seeds - Exclusive control over new plant variety - Contributions to conservation and development of resources - Production, marketing, and sale rights - Control over traditional knowledge Legal Protection Not universally recognized by all countries Provided through plant breeders' rights (PBR) or plant patents Purpose Ensuring farmers' control over genetic resources Encouraging investment in plant breeding and variety creation
- 77. Parameters Neem Patent Revocation Turmeric Patent Revocation Patent Holder US-based corporation (W.R. Grace and the Department of Agriculture of the United States) University of Mississippi Medical Center and two inventors Patent Details Patent (US 5,674,747) granted in 1997 for a method of controlling fungi using neem oil and related neem oil compositions Patent (US 5,401,504) granted in 1995 for the use of turmeric in wound healing Grounds for Revocation Lack of novelty and obviousness, as neem's fungicidal and medicinal properties were well-known and widely used in traditional systems Lack of novelty and obviousness, as turmeric's wound healing properties were already established in traditional medicine Prior Art Existing knowledge and uses of neem in traditional systems of agriculture and medicine Historical and traditional uses of turmeric for wound healing Public Opposition Extensive opposition from various groups and organizations, including indigenous communities, leading to a protracted legal battle Public outrage and concerns raised by Indian Council of Scientific and Industrial Research (CSIR) and traditional medicine experts Revocation Outcome The patent was revoked in 2005 after a lengthy legal battle and opposition efforts The patent was revoked in 1997 after widespread protests and opposition Impact Highlighted the issue of biopiracy and the need for better protection of traditional knowledge Raised awareness about the misappropriation of traditional knowledge and the importance of prior art in patent examinations Subsequent Developments The revocation spurred discussions on benefit-sharing, protection of traditional knowledge, and the role of international treaties Increased scrutiny and awareness regarding the patenting of traditional medicinal knowledge Lessons Learned and Awareness Increased awareness among communities and policymakers about the need for stronger safeguards against biopiracy Reinforced the importance of prior art and community involvement in the patenting process Case study of curcuma and neem
- 78. The Drugs and Cosmetics Act, 1940 regulates the AYUSH (Ayurveda, Siddha, Unani and Homoeopathic) drugs. Rules 151 to 159 of the Drugs and Cosmetics Rules, 1945 provide the regulatory provisions for grant of licenses to manufacture Ayurveda, Siddha and Unani drugs and promote their safety and quality. The standards of Ayurveda, Siddha and Unani drugs to be complied with are prescribed in Rule 168 of Drugs and Cosmetics Rules, 1945. Within the provisions of Drugs and Cosmetics Act, 1940, the Central Government can make or amend rules in this regard after consultation with or on the recommendation of the relevant Drugs Technical Advisory Board.
- 79. The technical enforcement or implementation related to the evaluation of AYUSH medicines is done by the following bodies: ™ •Ayurveda, Siddha, Unani Drugs Technical Advisory Board (ASUDTAB – which advises the government on issues related to the drugs, ™ •Ayurveda, Siddha, Unani Drugs Consultative Committee (ASUDCC – which advises the government on issues related to implementation, ™ •State licensing authorities who actually look after the testing process of AYUSH drugs to ascertain their purity, safety etc.
- 80. Schedule ‘Z’ The draft of Schedule Z as prepared by the sub-committee of Ayurveda, Siddha, Unani Drug Technical Advisory proposed to make clinical trials mandatory for all the patent or proprietary) medicines so as to examine the shelf life of ASU medicines. The revised version of the Schedule Z of the good clinical practices for clinical trials on ASU medicines as issued by the Department of AYUSH was faced vehement opposition from the AYUSH drug industry. The AYUSH drug industry considered the revised Schedule Z as highly unreasonable at the part of the ministry of AYUSH. The AYUSH drug industry suggested that implementation of the provisions of Schedule Z shall have a detrimental effect on the AYUSH formulation market.
- 82. Regulations in India (ASU, DTAB, DCC): Regulation provide protection to natural products users and nature also. It improves use of natural products and encourage research It establishes system for control of all activities like manufacturing, sales and use of herbal drug derived medicines. It gives penalty or punishment for persons who do not follow certain standards as per the rules. It prepares and amends laws, and enforce them to control natural products, thus provide safe and effective medicine.
- 90. REGULATIONS of HERBAL DRUGS/MEDICINE/TRADITIONAL MEDICINE in INDIA: In India herbal drugs are under the control of CDSCO (Central Drug Standard Control Organization) and Central authority under Central Government Ministry of Health and Family Welfare. Import, manufacturing, distribution and sale of herbal drugs/medicines are regulated by the following Acts and Rules in India. Drugs and Cosmetic Act 1940 Drugs and Cosmetic Rules 1945 Drugs and Magic Remedies Act, 1954 Narcotic and Psychotropic Substances Act 1985 Industries (Development Regulation) Act 1951 Trade and Merchandise Marks Act 1958 Indian Patents and Design Act 1970 Factories Act 1948.
- 91. Item Section Criteria Misbranded drugs 33 E ASU drugs are deemed to be misbranded: If colored or coated to conceal the damage or made appear better than therapeutic value If it is not labeled in prescribed manner If label or container accompanying drug bears any false claim or misleading. Adulterated drugs 33EE ASU drugs are deemed to be adulterated If it consists filthy, or decomposed material If prepared, packed or stored under insanitary conditions If its container contains any poisonous or deleterious substance colour other than one which is prescribed harmful or toxic substance If any substance mixed to reduce its quality or strength. Spurious drugs 33EEA ASU drugs are deemed to be spurious If it is sold or offered under another name. If it is an imitation or substitute for another drug If the label or container bears the name of an individual or company which is fictious. If it has been substituted by other drug REGULATIONS of HERBAL DRUGS/MEDICINE/TRADITIONAL MEDICINE in INDIA:
- 92. Regulation of manufacture for sale of ASU drugs. 33EEB. No person shall manufacture for sale or distribution, any ASU drug except in accordance with prescribed standards. Prohibition of manufacture and sale of certain ASU drug. 33EEC. No person shall manufacture ASU drugs which are: misbranded, adulterated or spurious patent or proprietary medicine, contravention to any of the provisions of the act Power of Central Government to prohibit manufacture of ASU drugs in public interest. 33EED. Central govt. can prohibit manufacture of ASU if: Drug involve any risk to human beings or animals Drug does not have the therapeutic value claimed Government Analysts. 33F. Central or State govt. can appoint any person with the prescribed qualification and do not have any financial interest in ASU drug. Inspectors. 33G. Central or State govt. can appoint any person with the prescribed qualification and do not have any financial interest in ASU drug. REGULATIONS of HERBAL DRUGS/MEDICINE/TRADITIONAL MEDICINE in INDIA:
- 93. Penalty for manufacture, sale, etc., of Ayurvedic, Siddha or Unani drug in contravention of this Chapter. 33I Manufacture for sale or for distribution of any ASU drugs deemed to be adulterated or without a valid license as required. Penalty for subsequent offences. 33J. Shall be punishable with imprisonment for a term not less than two years but which may extend to six years and with fine not less than 5000 INR. Confiscation. 33K. Any person convicted under the act, the respective stock of ASU drug can be confiscated. Cognizance of offences. 33M. No prosecution under this Chapter shall be instituted except by an Inspector. No Court inferior to that of a [Metropolitan Magistrate] or of a [Judicial Magistrate] of the first class shall try an offence punishable under this Chapter. Power of Central Government to make rules. 33N The Central Government may -after consultation with, or on the recommendation of, the Board- and after previous publication by notification in the Official Gazette, make rules for the purpose of giving effect to the provisions of this Chapter: Standards to be complied with in manufacture for sale or for distribution of Ayurvedic, Siddha and Unani Drugs. 168. Single drugs: The standards for identity, purity and strength as given in editions of Ayurvedic Pharmacopoeia of India. Asavas and Arishtas : The upper limit of alcohol as self generated alcohol should not exceed 12% v/v. REGULATIONS of HERBAL DRUGS/MEDICINE/TRADITIONAL MEDICINE in INDIA:
- 94. Application for license to manufacture Ayurvedic (including Siddha) or Unani drugs. 153 An application for the grant or renewal of a license to manufacture for sale any ASU drugs shall be made in Form 24-D to the Licensing Authority along with a fee of rupees sixty. Form of license to manufacture Ayurvedic (including Siddha) or Unani drugs: 154. Subject to the conditions of rule 157 being fulfilled, a license to manufacture for sale any ASU drugs shall be issued in Form 25-D. Loan License. 153-A. An application for the grant of renewal of a loan license to manufacture for sale of any ASU drugs shall be made in Form 25-E to the Licensing Authority along with a fee of rupees thirty. Certificate of award of G.M.P. of Ayurveda, Siddha and Unani Drugs. 155 B shall be issued to licensees who comply with the requirements of GMP of ASU drugs as laid down in Schedule T. REGULATIONS of HERBAL DRUGS/MEDICINE/TRADITIONAL MEDICINE in INDIA: Schedule Z: Pilot studies/Proof of Effectiveness instead of Phase I, II & III of Clinical Trials. Proposed changes have a direct bearing on Cost of New Product Development. Further, the Product Approval process would become very lengthy.
