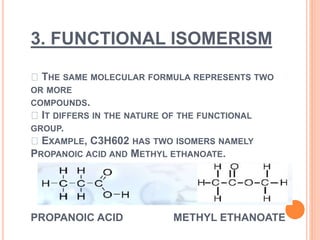
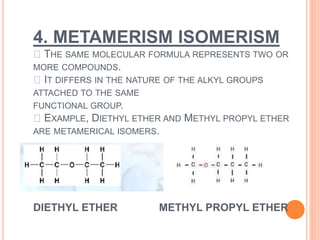
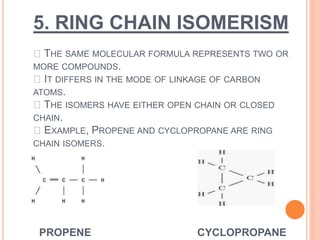
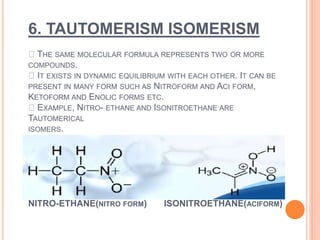
This document discusses the different types of isomerism:
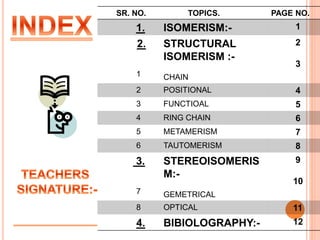
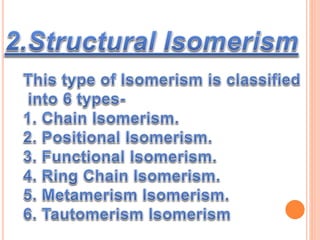
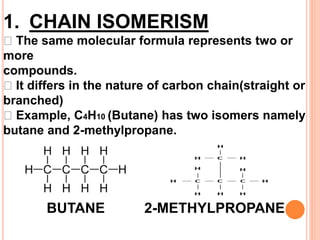
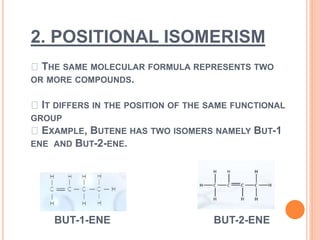
1. Structural isomerism includes chain, positional, functional, ring-chain, and metamerism isomers.
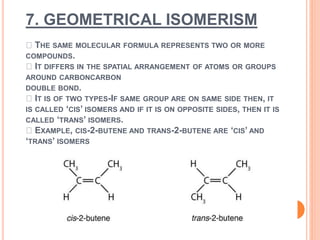
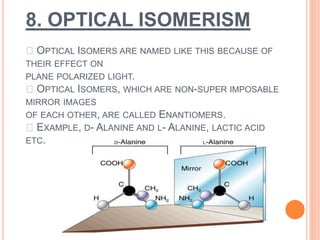
2. Stereoisomerism includes geometrical and optical isomers. Geometrical isomers differ in the spatial arrangement around a carbon-carbon double bond, while optical isomers are non-superimposable mirror images.
3. Examples are provided for each type of isomerism to illustrate the differences between isomers of the same molecular formula.