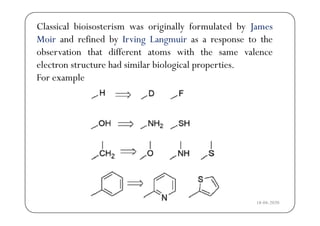
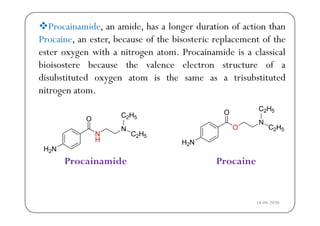
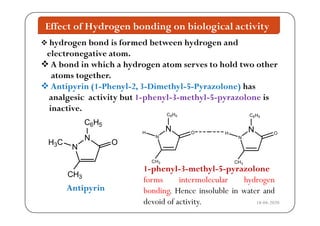
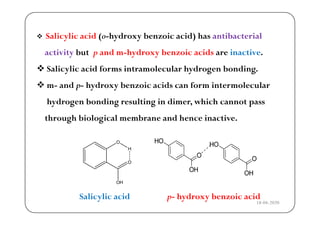
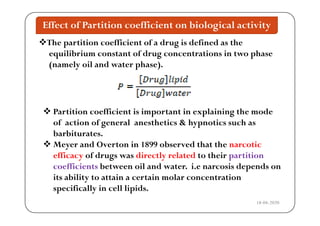
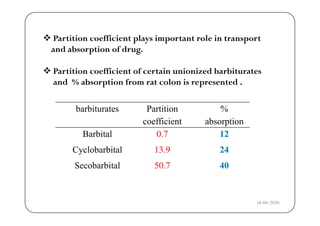
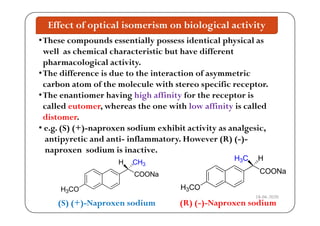
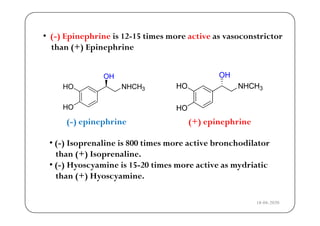
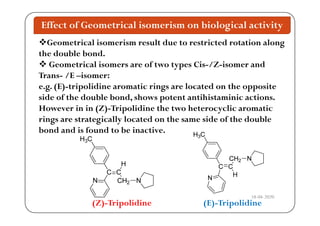
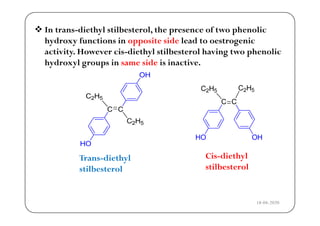
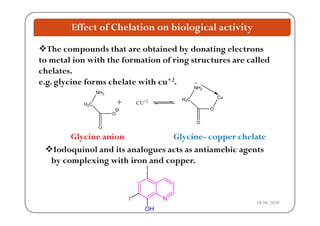
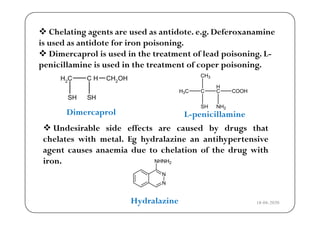
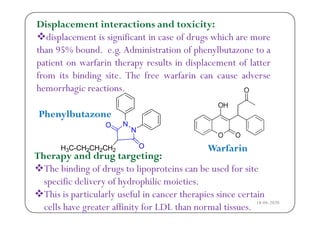
The document discusses various factors affecting biological activity related to drugs, including hydrogen bonding, partition coefficients, optical and geometrical isomerism, chelation, protein binding, ionization, solubility, and bioisosterism. It highlights the importance of these properties in drug design and their implications for therapeutic efficacy. Additionally, it provides examples of specific compounds to illustrate how these factors influence the biological effects of drugs.

![Effect of ionisation on biological activity
The majority of the drugs are either weak acid (for example,
acetylsalicylic acid [aspirin] or weak base (for example, procaine).y y [ p ] ( p , p ).
Acidic drugs can give proton and basic drug can accept a
proton to ionise.p
Amphoteric drugs containing both acidic and basic drug can
give and accept proton.g p p
The dissociation of acetylsalicylic acid, a weak acid, could be
represented below. In this equilibrium, acetylsalicylic acid acts
O COCH3 O COCH
p . q , y y
as an acid, because it donates a proton.
O COCH3
OH
O
+ H2O
O COCH3
O
O
+ H3O
18-04-2020
O O](https://image.slidesharecdn.com/physicochemicalfactoraffectingbiologicalactivity-200418170257/85/Effect-of-physicochemical-factors-on-biological-activity-Medicinal-Chemistry-14-320.jpg)