Embed presentation
Downloaded 22 times

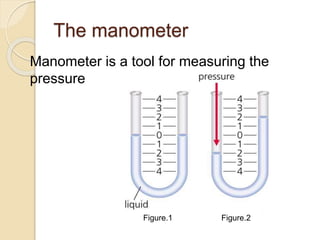
![Manometer depends on 3 factors;
1. the Height of the column of the fluid
[H]
2. The density of the fluid [ρ]
3. The gravitaional constant [g] which
equal 9.81 m/s2
So the pressure in manometer = H x ρ x
g](https://image.slidesharecdn.com/pressure-150901173347-lva1-app6891/85/stem-Pressure-4-320.jpg)
![The barometer
Measurement tool for pressure of gases
[atmospheric pressure]
It depends on the direct proportional
between the pressure of the air
[atmospheric pressure] and the
height of the column of the mercury.](https://image.slidesharecdn.com/pressure-150901173347-lva1-app6891/85/stem-Pressure-5-320.jpg)
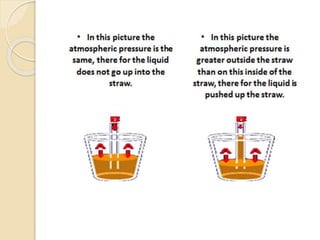
![The straw
The straw depends on the idea of the
barometer that;
[as the atmospheric pressure decreases
the height of the column of liquid
increases]](https://image.slidesharecdn.com/pressure-150901173347-lva1-app6891/85/stem-Pressure-6-320.jpg)

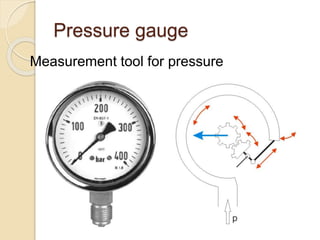

![Gauge pressure Vs total
pressure
Total pressure =
the pressure on the surface of the liquid
+ the atmospheric pressure [gauge
pressure]
Gauge pressure =
total pressure – the pressure on the
surface of the liquid](https://image.slidesharecdn.com/pressure-150901173347-lva1-app6891/85/stem-Pressure-11-320.jpg)

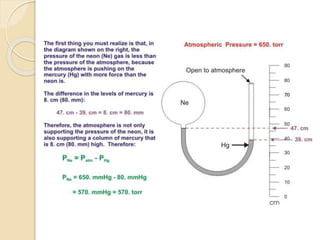
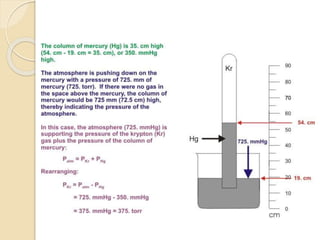

This document discusses different tools used to measure pressure, including manometers, barometers, and pressure gauges. It explains that a manometer measures pressure using the height of a fluid column based on factors like the fluid's density and gravity. A barometer also relies on the direct relationship between atmospheric pressure and the height of a mercury column. The application of straw demonstrates how decreasing air pressure causes the liquid level in the straw to rise. Finally, it notes that a pressure gauge uses a needle to indicate the pressure measured by a fluid's flow through a pump.



![Manometer depends on 3 factors;
1. the Height of the column of the fluid
[H]
2. The density of the fluid [ρ]
3. The gravitaional constant [g] which
equal 9.81 m/s2
So the pressure in manometer = H x ρ x
g](https://image.slidesharecdn.com/pressure-150901173347-lva1-app6891/85/stem-Pressure-4-320.jpg)
![The barometer
Measurement tool for pressure of gases
[atmospheric pressure]
It depends on the direct proportional
between the pressure of the air
[atmospheric pressure] and the
height of the column of the mercury.](https://image.slidesharecdn.com/pressure-150901173347-lva1-app6891/85/stem-Pressure-5-320.jpg)
![The straw
The straw depends on the idea of the
barometer that;
[as the atmospheric pressure decreases
the height of the column of liquid
increases]](https://image.slidesharecdn.com/pressure-150901173347-lva1-app6891/85/stem-Pressure-6-320.jpg)




![Gauge pressure Vs total
pressure
Total pressure =
the pressure on the surface of the liquid
+ the atmospheric pressure [gauge
pressure]
Gauge pressure =
total pressure – the pressure on the
surface of the liquid](https://image.slidesharecdn.com/pressure-150901173347-lva1-app6891/85/stem-Pressure-11-320.jpg)


