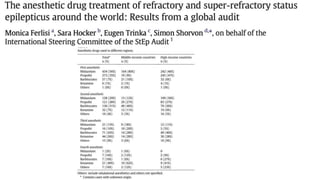
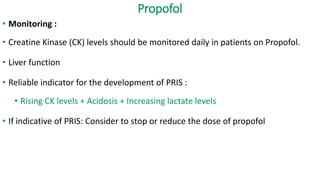
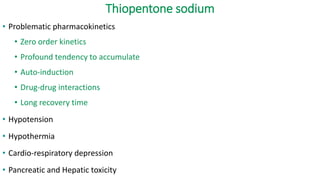
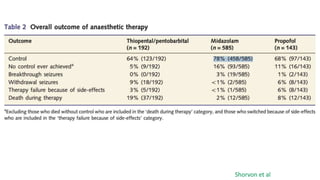
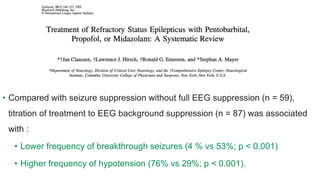
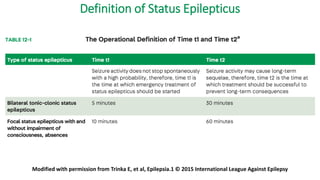
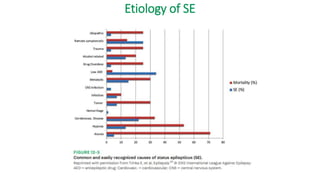
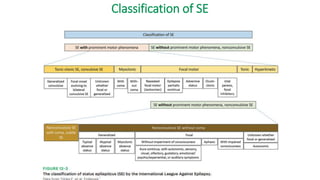
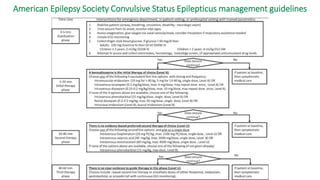
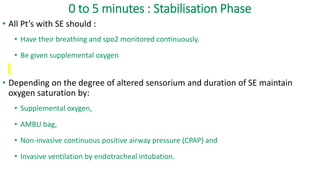
The document discusses guidelines for the management of status epilepticus, dividing treatment into phases from 0-5 minutes for stabilization, 5-20 minutes for initial benzodiazepine therapy, 20-40 minutes for a second line anti-seizure medication like fosphenytoin, valproic acid, or levetiracetam, and 40-60 minutes for third line therapy if needed. Rapid treatment and identification of any underlying causes is important to prevent complications and mortality from ongoing seizures.














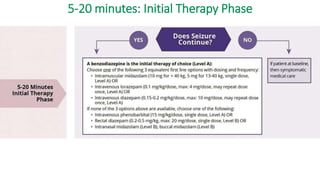
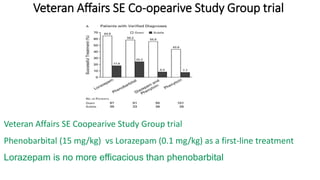
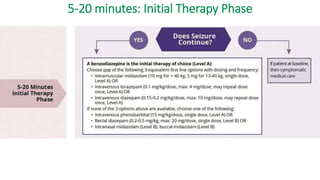
![5-20 minutes: Initial Therapy Phase
• Nine RCTs addressed the efficacy of initial therapy
• Three class I
• One class II and
• Five class III
• The following are the class I trials :
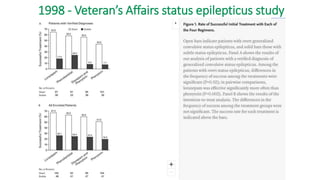
• 1998 - Veteran’s Affairs status epilepticus study
• 2001 - Comparison of Lorazepam, Diazepam, and Placebo for the Treatment of Out-of-Hospital
Status Epilepticus [NEJM]
• 2012 - RAMPART trial](https://image.slidesharecdn.com/statusepilepticus-management-231124100231-9f9ab862/85/status-epilepticus-management-23-320.jpg)




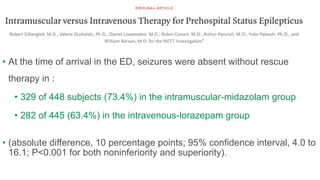
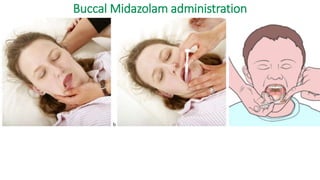
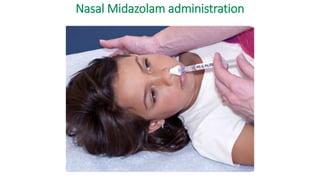
![• Dosage:
• Adults and children with an estimated body weight of more than 40 kg:
• 10 mg of IM Midazolam followed by IV placebo [or] IM placebo followed
by 4 mg of IV Lorazepam.
• In children with an estimated weight of 13 to 40 kg :
• 5 mg of IM Midazolam or 2 mg of IV Lorazepam.](https://image.slidesharecdn.com/statusepilepticus-management-231124100231-9f9ab862/85/status-epilepticus-management-29-320.jpg)