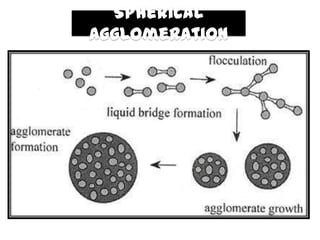
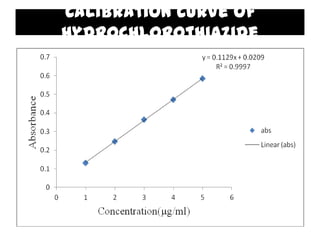
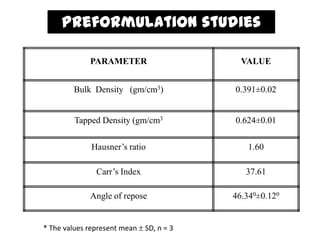
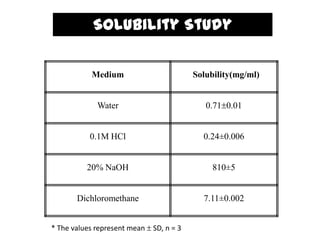
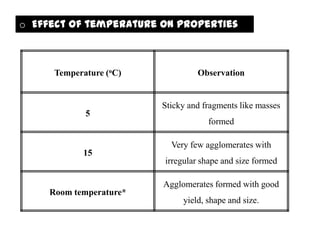
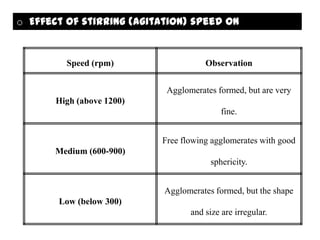
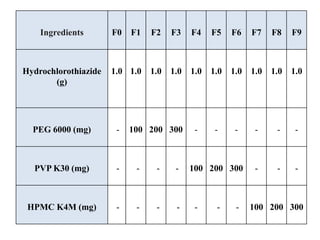
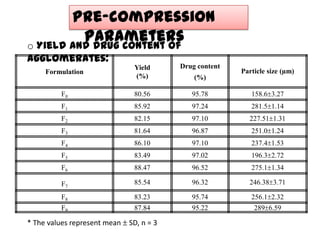
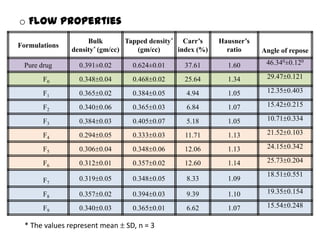
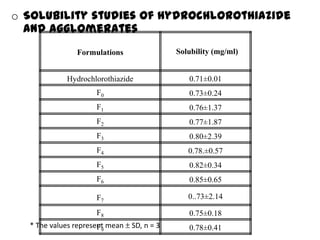
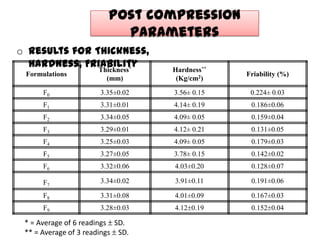
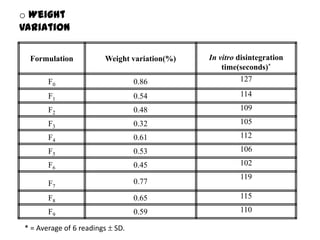
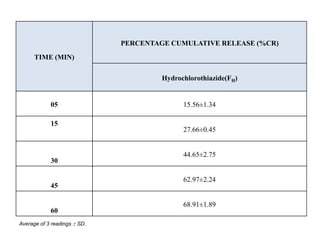
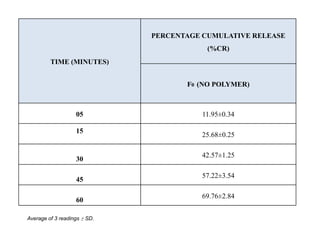
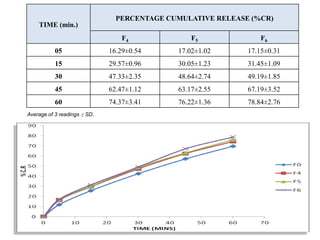
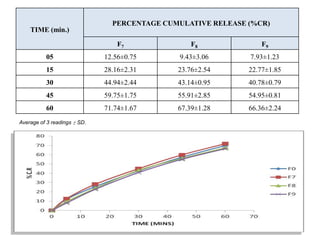
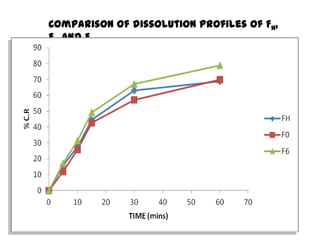
The document summarizes the preparation and evaluation of spherical agglomerates of hydrochlorothiazide using the neutralization method. Various formulation and process parameters were optimized to obtain spherical agglomerates with improved flow and compression properties compared to the drug alone. The optimized formulation F6 containing PVP K30 showed significantly improved solubility and dissolution profile of the drug. In conclusion, the spherical agglomeration technique helped render the poorly compressible drug suitable for direct compression into tablets.